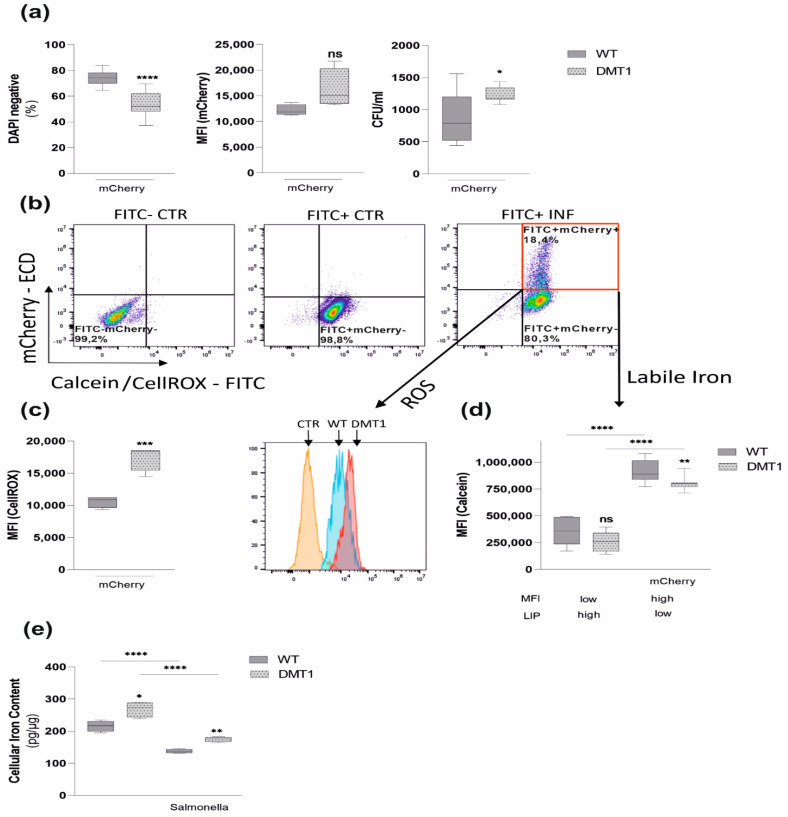
Figure 4.
DMT1 modulates iron availability in control and infected macrophages. Macrophages were infected with red fluorescent protein expressing Salmonella strains (mCherry) for 6 h. (a) The percentage of DAPI-negative cells indicating macrophage viability (n = 3) and mean fluorescence intensity (MFI) of mCherry infected macrophages (n = 3) suggesting mCherry proliferation. (b) Samples were stained either with CellROX Green (c) to determine oxidative stress or Calcein-AM (d) to measure LIP. A representative experiment with CellROX is shown using the following template: unstained control (CTR, FITC-mCherry−), stained control (FITC+mCherry−), stained infected (INF, FITC+mCherry+) BMDMs. (c) Reactive oxygen species (ROS) among mCherry (FITC+mCherry+)-containing macrophages were determined by flow cytometry. Data from 3 experiments are shown. Representative histogram including the FITC+mCherry-control normalized to mode is shown. (d) Calcein quench experiments were performed: DMT1 decreased the labile iron pool in infected macrophages (FITC+mCherry+) and uninfected macrophages (FITC+mCherry−), as evidenced by increasing Calcein-mediated mean fluorescence intensity (MFI). (e) Total iron content of control and infected macrophages was measured by atomic absorption (n = 2); Duplicates or triplicates from at least two independent experiments were compared by two-tailed unpaired t-test (two groups) or analysis of variance (ANOVA) using Bonferroni correction (more than two groups); n = mice per group. Box plots display whiskers with the minimum to maximum. Statistical significance * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001, ns, no significance of differences; DMT1: DMT1 fl/flLysMCre(+), WT: wildtype, mCherry: red fluorescent protein (RFP)-expressing Salmonella.