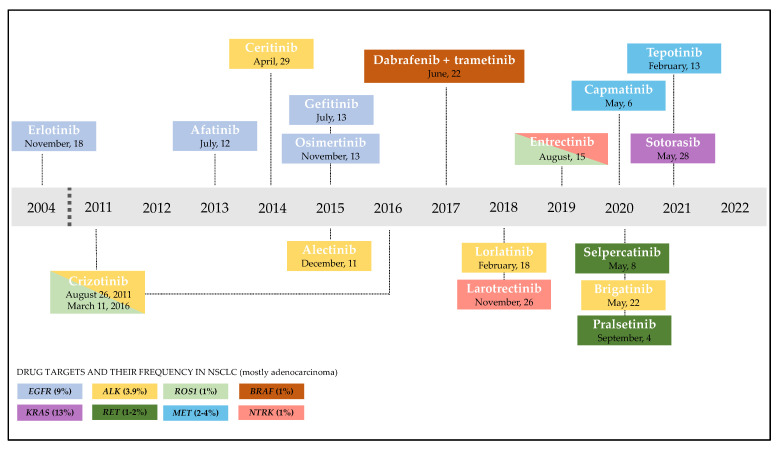
Figure 1.
Timeline of FDA approval of targeted therapies for NSCLC (the colors are matched between driver alteration and targeted agent). The identification of actionable biomarkers led to significant progress in the treatment of NSCLC. EGFR alterations are detected in approximately 9% of NSCLC patients [6,7] and in the last two decades several agents targeting sensitive mutations received approval from the FDA. The first–generation EGFR–TKI inhibitors, erlotinib and gefitinib, received FDA approval for the treatment of advanced NSCLC in November 2004 and July 2015, respectively. Afatinib is the most studied second–generation inhibitor and received approval in July 2013. Two years later, the third–generation TKI-inhibitor, osimertinib, was initially approved for the treatment of EGFR–T790M mutation positive NSCLC, then in April 2018 it received approval as a first–line treatment for EGFR mutated NSCLC. ALK fusion–positive tumors account for 3.9% of NSCLC adenocarcinomas [8]. Several targeted drugs are available for this subset of patients: the first–generation drug crizotinib was approved in August 2011 and then the FDA expanded its use to treat ROS1–positive patients, a rare subgroup accounting for approximately 1% [9]. Second–generation ALK inhibitors, ceritinib, alectinib and brigatinib, were approved by the FDA between April 2014 and May 2020. The third–generation inhibitor lorlatinib received approval in 2018 for pretreated ALK–positive patients, and later in 2021 for the first–line setting. In June 2017, the FDA approved a combination therapy of dabrafenib and trametinib for BRAFV600E mutation–positive metastatic NSCLC, accounting for 1% of lung cancer patients [10]. NTRK is found in 1% of NSCLC [19,20]. Larotrectinib is a specific NTRK inhibitor approved in 2018 and represents the second tissue–agnostic FDA approval for the treatment of cancer Entrectinib received approval in August 2019 for both treatment of NTRK and ROS1- positive NSCLC. In the last two years, major progress has been made: in 2020 the FDA approved the targeted agents selpercatinib and pralsetinib for RET fusion–positive NSCLC (1–2%) [5,21]; capmatinib and tepotinib received FDA approval for NSCLC harboring a METex14 skipping mutation (2–4%) [22,23] in May 2020 and February 2021, respectively; sotorasib was approved in May 2021 for the treatment of KRASG12C mutated NSCLC (approximately 13%) [24] in patients who have received at least one prior systemic therapy.