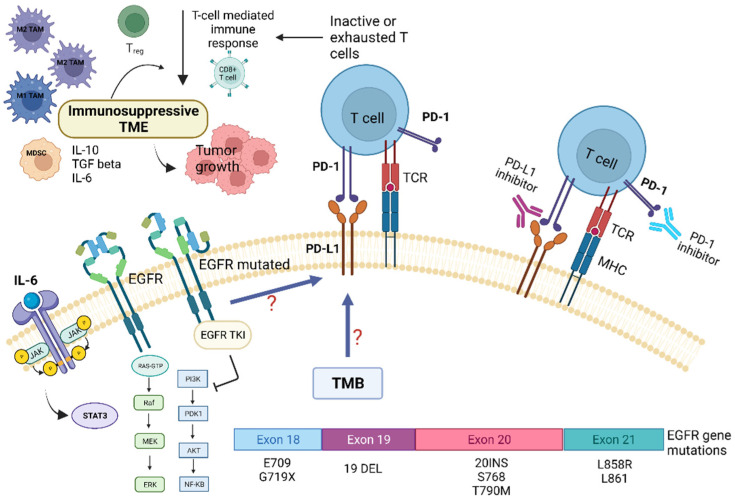
Figure 2.
Role of tumor microenvironment in EGFR-mutated NSCLC in influencing resistance pathways to targeted TKI treatment and potential targets for immunotherapy. EGFR mutations are associated with immunosuppressive TME, lower tumor-mutation burden (TMB), and increased PD-L1 expression. EGFR mutations may promote cancer immune escape through modulation of the PD-1/PD-L1 pathway, which in turn determine T-cells inactivity and/or exhaustion. This also leads to EGFR-TKI resistance. In addition, EGFR mutations influence several TME components, such as tumor-infiltrating lymphocytes (TILs), Tregs, MDSCs, TAMs, and immunoregulatory/proinflammatory cytokines, i.e., IL-6. The latter, through the activation of the STAT-3 intracellular pathway, contribute to tumor growth and resistance to targeted therapies. Abbreviations: AKT—serine-threonine kinase; EGFR, epidermal growth factor receptor; ERK—extracellular signal-regulated kinase; IL—Interleukin; JAK—Janus kinase; MHC—major histocompatibility complex; MEK—mitogen-activated protein kinase; MDSC—myeloid-derived suppressor cells; NF-kB, nuclear factor kappa B; PI3K—phosphatidylinositol-4,5-bisphosphate 3-kinase; PD-1—programmed death; PD-L1—programmed death ligand-1; TKI—Tyrosine kinase inhibitors; Treg—regulatory T-cell; STAT3—signal transducer and activator of transcription 3; TCR—T-cell receptor; TMB—tumor mutational burden. Created with BioRender.com (https://biorender.com/, accessed on 17 May 2022).