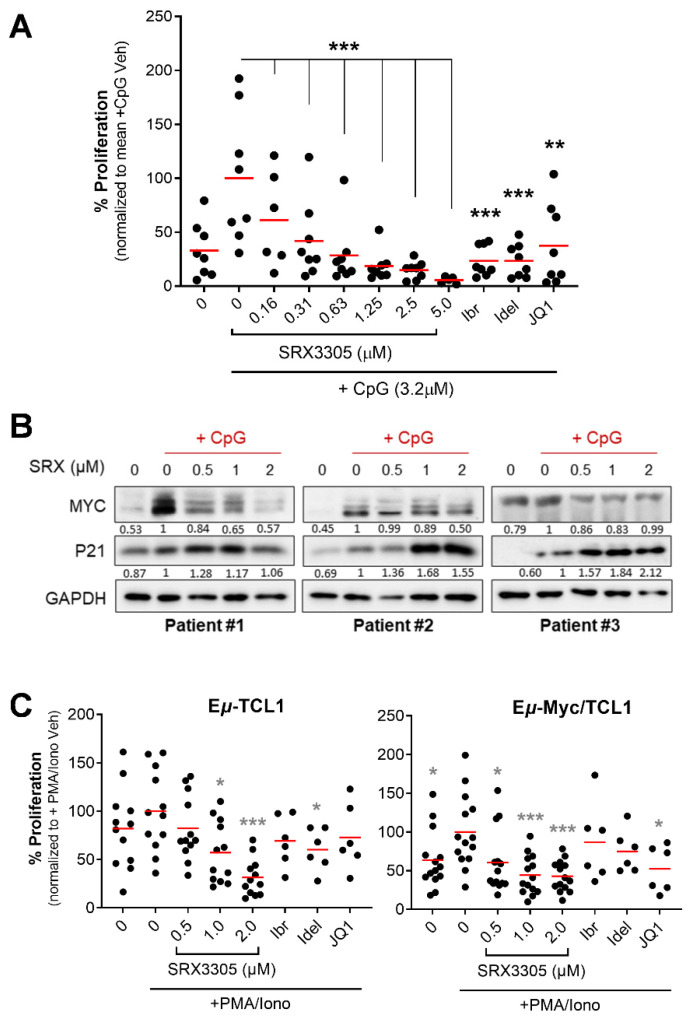
Figure 3.
Antitumor effects of SRX3305 in primary CLL samples. (A) Patient-derived CLL cells (n = 8) were treated with the indicated concentrations of SRX3305; 1 μM of ibrutinib (Ibr), idelalisib (Idel), or JQ1; or DMSO vehicle. They were then stimulated with CpG oligodeoxynucleotides (+CpG; 3.2 μM) for 48 h. Proliferation was assessed via MTS assay, and results are given as % proliferation normalized to mean stimulated vehicle (+CpG Veh). Red lines indicate average values. p values indicate significance vs. stimulated vehicle. (B) Patient-derived CLL cells (n = 6) were treated with DMSO vehicle or the indicated SRX3305 concentrations for 4 h in the presence of CpG oligonucleotides (+CpG, 3.2 µM). The protein expression of MYC and P21 was determined by immunoblot analysis. GAPDH serves as a loading control. Representative immunoblots from three patients are shown. Numbers below the bands represent densitometric quantification relative to loading control and normalized to +CpG Veh. (C) Lymphocytes isolated from spleens of terminally diseased Eμ-TCL1 (n = 6–12) or Eμ-Myc/TCL1 (n = 6–14) mice were stimulated with 1× PMA/Ionomycin (+PMA/Iono) and treated with DMSO vehicle or increasing concentrations of SRX3305 as indicated, or 1 μM Ibr, Idel, or JQ1. Proliferation was assessed via MTS assay 48 h later, and results are given as % proliferation normalized to mean stimulated vehicle (+PMA/Iono Veh). Red lines indicate averages. p values indicate significance vs. stimulated vehicle. * p < 0.05, ** p < 0.01, *** p < 0.001.