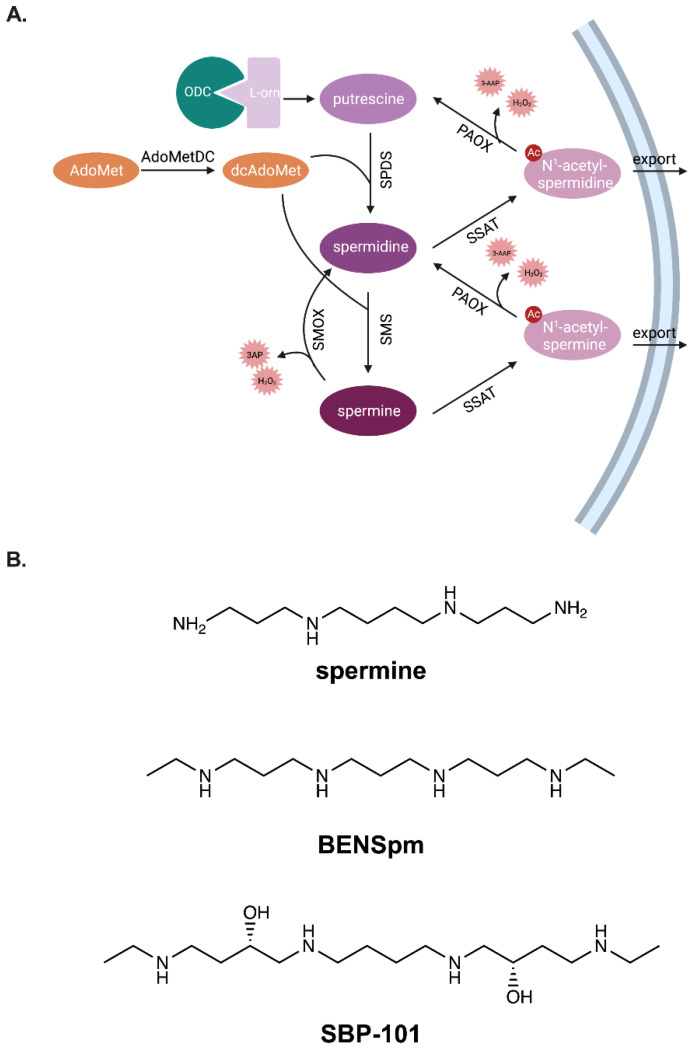
Figure 1.
Polyamine metabolism is regulated through coordinated biosynthesis, catabolism, and transport. Ornithine decarboxylase (ODC) is a rate-limiting enzyme in polyamine biosynthesis (A). It catalyzes the formation of putrescine from L-ornithine. Spermidine synthase (SPDS) catalyzes the formation of spermidine from putrescine, and spermine synthase (SMS) assists in producing spermine from spermidine. Both reactions require decarboxylated adenosylmethionine produced by S-adenosylmethionine decarboxylase (AdoMetDC), the second rate-limiting enzyme in polyamine biosynthesis. Spermine oxidase (SMOX) directly catabolizes spermine to spermidine and produces the toxic byproducts, 3-aminopropanal and hydrogen peroxide. Alternatively, spermine can be acetylated by spermidine/spermine N1-acetltransferase (SSAT) to form N1-acetyl-spermine, which can be exported from the cell or further oxidized by polyamine oxidase (PAOX) to form spermidine. Spermidine can be catabolized to putrescine in a similar manner, forming N1-acetyl-spermidine as an intermediary. Analogues of the polyamine spermine compete for uptake into cells, and their accumulation influences polyamine metabolism (B). The well-characterized polyamine analogue, N1,N11-bisethylnorspermine, influences ODC, AdoMetDC, SSAT, and SMOX to affect polyamine metabolism. The effects of the spermine analogue, SBP-101, on polyamine metabolism are addressed in this manuscript.