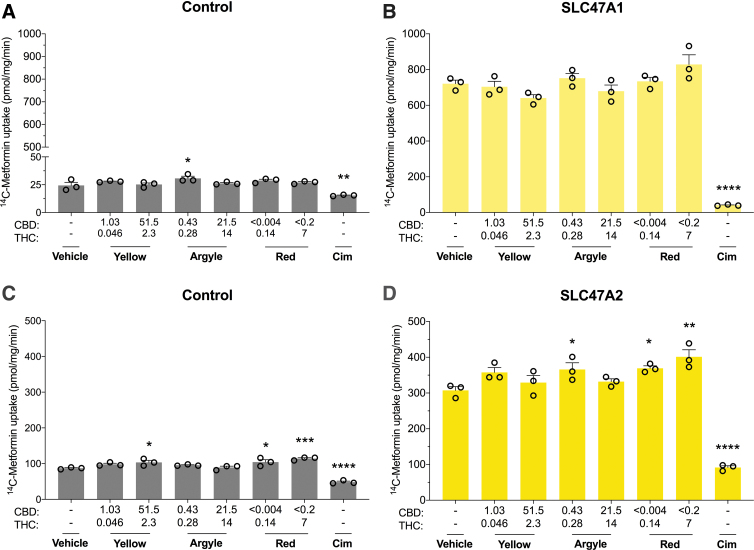
FIG. 7.
The three cannabis-based products did not inhibit SLC47A1 or SLC47A2 transporters. Cells expressing human multidrug and toxin extrusion transporters were used to screen the inhibitory potential of three cannabis-based products. Average uptake of 14C-Metformin (20 μM) into cells (A) transfected with control vector or (B) expressing SLC47A1 was quantified by liquid scintillation counting. Spectrum Yellow (Yellow), Tweed Argyle (Argyle), and Spectrum Red (Red) cannabis-based products were applied with final CBD and THC concentrations (μM) depicted (Cim, 50 μM). None of the cannabis-based products affected SLC47A1 transport. Error bars represent SEM, with n=3 per group. Average uptake of 14C-Metformin (90 μM) into cells (C) transfected with control vector or (D) expressing SLC47A2 was quantified by liquid scintillation counting (Cim, 50 μM). In control cells, uptake of 14C-Metformin increased in the presence of the cannabis-based products (*p<0.05, ***p<0.001, ****p<0.0001 compared with vehicle; one-way ANOVA followed by Dunnett's post hoc). Error bars represent SEM, with n=3 per group. Spectrum Red and low-dose Tweed Argyle slightly increased uptake of 14C-Metformin (*p<0.05, **p<0.005, ****p<0.0001 compared with vehicle; one-way ANOVA followed by Dunnett's post hoc). Error bars represent SEM, with n=3 per group. Cim, cimetidine.