Figure 1.
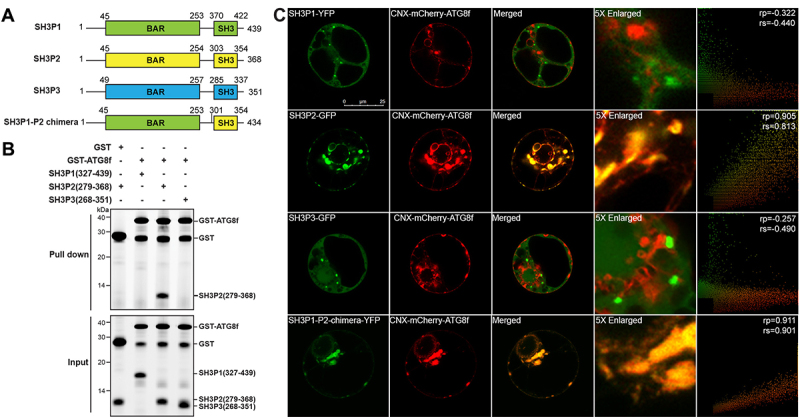
SH3P2, but not SH3P1 and SH3P3, binds to Arabidopsis ATG8f. (A) Domain organization of Arabidopsis SH3Ps proteins. SH3P1, SH3P2 and SH3P3 all contain an N-terminal BAR domain and a C-terminal SH3 domain. (B) GST affinity-isolation assay. 1 µM SH3 domain protein of SH3P1, SH3P2 and SH3P3 was mixed with 1 µM GST-ATG8f and incubated with glutathione resin (upper panel) respectively. The bound protein was eluted with 10 mM glutathione, and then pre-stained by Instant-Bands and analyzed by SDS-PAGE (lower panel). Our results suggested that GST-ATG8f only interacted with the SH3 domain of SH3P2 but not with the SH3 domains of SH3P1 and SH3P3. (C) SH3Ps-GFP/YFP, or SH3P1-P2-chimera-YFP, and CNX-mCherry-ATG8f were transiently co-expressed in Arabidopsis protoplasts. Confocal analysis showed that SH3P2, but not SH3P1 and SH3P3, colocalized with CNX-mCherry-ATG8f. Similar results were obtained from three different independent experiments. The right column shows the scatterplot images obtained from ImageJ with the PSC colocalization plug-in. The linear Pearson correlation coefficient (rp) and the nonlinear Spearman correlation coefficient (rs) indicate the extent of colocalization with the value of +1.0 for complete colocalization.
