Figure 4.
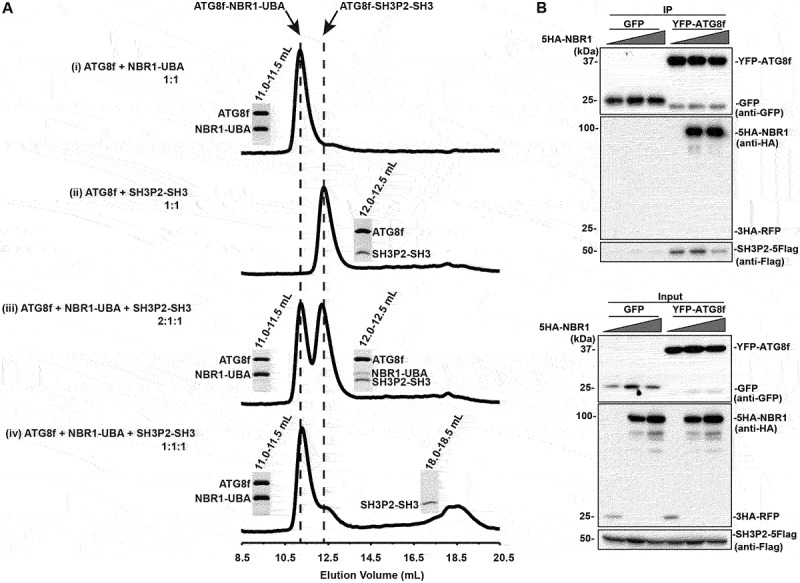
NBR1-UBA outcompetes SH3P2-SH3 for ATG8 binding. (A) ATG8f was mixed with NBR1-UBA and/or SH3P2-SH3 and injected into a Superdex 75 10/300 gel filtration column pre-equilibrated in 1X PBS, 5 mM DTT. The fractions corresponding to the peaks were resolved by SDS–PAGE followed by Coomassie Brilliant Blue staining. (i) When 40 µM ATG8f was mixed with 40 µM NBR1-UBA in 1:1 molar ratio, they formed an ATG8f-NBR1-UBA complex that eluted at ~11.2 ml. (ii) When 40 µM ATG8f was mixed with 40 µM SH3P2-SH3 in 1:1 molar ratio, they formed an ATG8f-SH3P2-SH3 complex that eluted at ~12.3 ml. (iii) When 80 µM ATG8f was mixed with 40 µM NBR1-UBA and SH3P2-SH3 in 2:1:1 molar ratio, both peaks at ~11.2 and ~12.3 ml were present, suggesting both ATG8f-NBR1-UBA and ATG8f-SH3P2-SH3 were formed. (iv) When 40 µM ATG8f was mixed with 40 µM NBR1-UBA and SH3P2-SH3 in 1:1:1 molar ratio, majority of ATG8f formed a complex with NBR1-UBA. On the other hand, a peak at ~18.5 ml representing the free form of SH3P2-SH3 was observed. (B) Increasing amount of 5HA-NBR1 (0:1:3) was transiently co-expressed with SH3P2-5Flag and YFP-ATG8f or GFP in Arabidopsis protoplasts for 12 h, followed by the GFP trap assay. 3HA-RFP was used as a control. The resulting immunoprecipitation and cell lysate were analyzed by immunoblotting using anti-Flag, anti-HA or anti-GFP antibodies respectively.
