Figure 6.
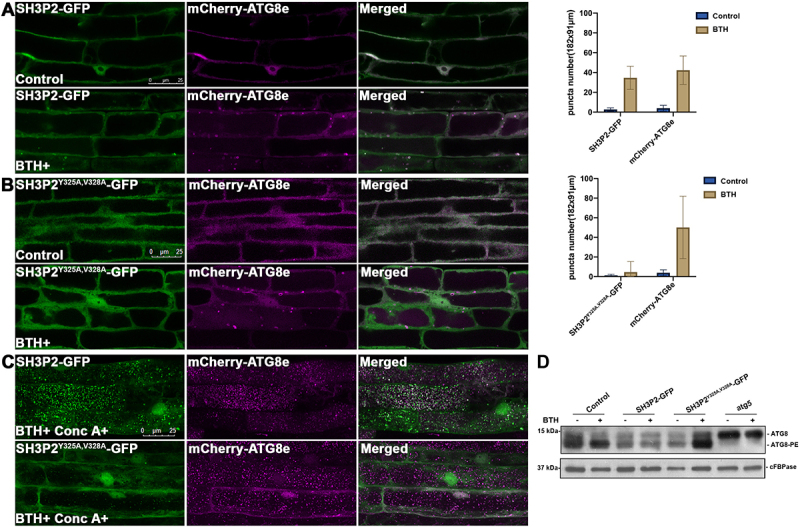
The AIM-like motif is essential for SH3P2 recruitment to the phagophore membrane upon autophagic induction. (A) 4-d-old seedlings were transferred to medium with or without BTH for 6 h respectively. Upon BTH treatment, SH3P2-GFP was redistributed to puncta and ring-like structures that overlapped with mCherry-ATG8e in transgenic plants expressing both SH3P2-GFP and mCherry-ATG8e. Quantification of the puncta labeled by SH3P2 or mCherry-ATG8e were obtained from more than 10 individual seedlings (error bars ±SD). (B) Upon BTH treatment, no co-localization of SH3P2-GFP-labeled structures with mCherry-ATG8e was observed in transgenic plants expressing SH3P2Y325A,V328A-GFP and mCherry-ATG8e, suggesting that the SH3P2Y325A,V328A mutation impaired recruitment of SH3P2-GFP to autophagosomes. Quantification of the puncta labeled by SH3P2Y325A,V328A-GFP or mCherry-ATG8e were obtained from more than 10 individual seedlings (error bars ±SD). (C) SH3P2-GFP, and SH3P2Y325A,V328A-GFP seedlings were incubated in medium with/without BTH and Conc A treatment for 6 h respectively. Autophagic bodies upon BTH and Conc A treatment labeled by GFP signals were significantly downregulated in SH3P2Y325A,V328A-GFP transgenic plants. (D) Immunoblot detection of the ATG8 lipidation level in wild-type, SH3P2, SH3P2Y325A,V328A-GFP and atg5 plants. 5-d-old wild type, SH3P2-GFP, SH3P2Y325A,V328A-GFP and atg5 seedlings were incubated in medium with/without BTH treatment for 6 h respectively. Membrane fractions were subjected to immunoblot analysis with ATG8 antibodies. Immunoblotting with cFBPase antibodies was used as a loading control.
