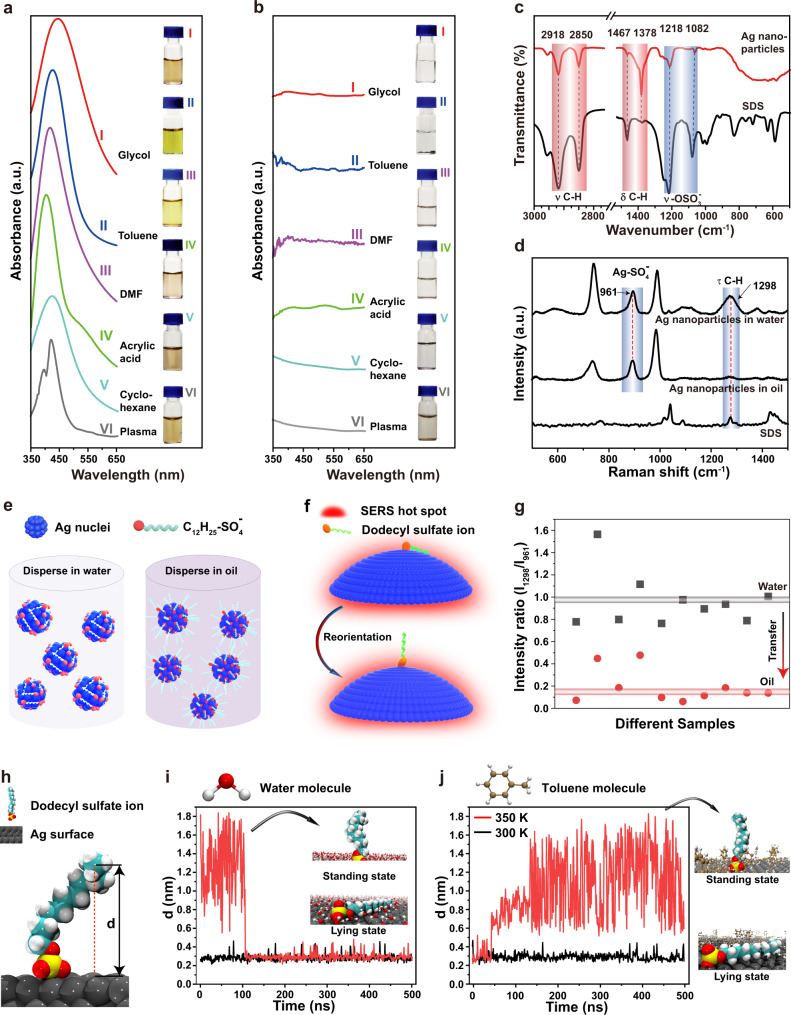
Fig. 3. Rotatable surface ligands endowed the smart Ag nanoparticles with anomalous dispersive properties.
a The absorption spectra of the smart Ag nanoparticles dispersed in different organic liquids without any chemical modification after electrodeposition in aqueous solution. b Ag nanoparticle prepared by wet chemical method in water immediately aggregated in organic liquids. Insets in (a), (b) are photos of the dispersive status of the Ag nanoparticles in different liquids. c FTIR spectra of the smart Ag nanoparticles and SDS powder. d SERS spectra of the smart Ag nanoparticles dispersed in water and oil and the SDS powder. e Smart Ag nanoparticles exposing hydrophilic sulfate head in water and hydrophobic alkyl chain in oil. f Schematic of the orientation change of the dodecyl sulfate surface ligands when transfer the smart Ag nanoparticle from water to oil, as well as the mechanism to monitor the orientation change by SERS. g The evolution of the intensity ratio between the 1298 cm−1 (from the dodecyl tail) and the 961 cm−1 (from sulfate head) SERS peaks when transfer the smart Ag nanoparticles from water to oil (e.g., toluene). The data were collected from 10 different samples. h The vertical distance between the end carbon atom in the dodecyl chain and the Ag surface was defined as d. i MD simulation of the d variation when the ligand at the lying state (black curve) and the standing state (red curve) exposed to water. Inset: the ligand at the standing state and the lying state in water captured from Supplementary Movie 3. j MD simulation of the d variation when the ligand at lying state exposed to toluene at 300 K (black curve) and 350 K (red curve). Inset: the ligand at the standing state and the lying state in toluene captured from Supplementary Movie 4. Note: In the insets, only the solvent molecules on the Ag surface were shown, while the ligand was fully immersed in the solvent in MD simulations.