Abstract
Ischemic stroke is a primary cause of morbidity and mortality worldwide. Beyond the approved thrombolytic therapies, there is no effective treatment to mitigate its progression. Drug repositioning combinational therapies are becoming promising approaches to identify new uses of existing drugs to synergically target multiple disease-response mechanisms underlying complex pathologies. Here, we used a systems biology–based approach based on artificial intelligence and pattern recognition tools to generate in silico mathematical models mimicking the ischemic stroke pathology. Combinational treatments were acquired by screening these models with more than 5 million two-by-two combinations of drugs. A drug combination (CA) formed by ceruletide and alpha-1 antitrypsin showing a predicted value of neuroprotection of 92% was evaluated for their synergic neuroprotective effects in a mouse pre-clinical stroke model. The administration of both drugs in combination was safe and effective in reducing by 39.42% the infarct volume 24 h after cerebral ischemia. This neuroprotection was not observed when drugs were given individually. Importantly, potential incompatibilities of the drug combination with tPA thrombolysis were discarded in vitro and in vivo by using a mouse thromboembolic stroke model with t-PA-induced reperfusion, revealing an improvement in the forepaw strength 72 h after stroke in CA-treated mice. Finally, we identified the predicted mechanisms of action of ceruletide and alpha-1 antitrypsin and we demonstrated that CA modulates EGFR and ANGPT-1 levels in circulation within the acute phase after stroke. In conclusion, we have identified a promising combinational treatment with neuroprotective effects for the treatment of ischemic stroke.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01203-0.
Keywords: Ischemic stroke, Neuroprotection, Ceruletide, Alpha-1 antitrypsin, Combinational therapy
Introduction
Ischemic stroke is among the leading causes of morbidity and mortality worldwide [1]. The only current therapy for ischemic stroke aims at restoring cerebral blood flow by removing the obstructive clot, via the intravenous administration of tissue plasminogen activator (t-PA) or tenecteplase (TNK) or through mechanical thrombectomy using stent retriever or aspiration devices [2, 3]. Despite the effectiveness of reperfusion strategies, their short therapeutic window and some potential side effects preclude offering those therapies to all stroke patients. Therefore, there is still an important need to find alternative stroke therapies to rescue brain tissue from the ischemic injury that could be implemented to the largest number of patients possible. In this regard, an increasing number of pre-clinical and clinical studies have now moved their focus of attention to the pre-hospital phase or the early post-arrival emergency department [4]. In such scenario, patients could be treated even before the stroke diagnosis is confirmed by neuroimaging techniques in the medical facility and under the hypothesis that treatments might be most effective when initiated as soon as possible after stroke onset.
Since ischemic stroke is well-known to be a multifactorial and heterogeneous disease, there is growing awareness that targeting a single pathological activated pathway might be insufficient to impact on the progression of the disease [5], a fact that could explain the systematic failure of translation into clinics of neuroprotectants tested so far [6–8]. In this sense, a global understanding of the different pathways that are disturbed in this multifactorial disorder is becoming critical to successfully find new therapeutic agents to reverse stroke progression [9]. Systems biology has emerged as a new discipline to precisely decipher this complexity. To that end, it considers organisms as sophisticated network-based maps of highly interrelated molecules and connected biological pathways. Systems biology provides insights into those relevant elements whose differential activity is associated with the disease, and simulates the behavioral response of the pathology to the modulation of any disease-related mediators [10].
Approaches based on systems biology are also particularly suited for drug repositioning strategies [11]. The integration of current biological and clinical knowledge with data on therapeutic responses facilitates the identification of new uses for existing drugs as promising healing agents outside their original medical indication. In fact, there are already examples of drug repositioning approaches in the field of ischemic stroke applying the concept of systems biology [12, 13]. More importantly, systems biology–based drug repositioning strategies are also making significant contributions to the identification of combinational treatment approaches, in which two or more therapeutic agents are combined to synergically target multiple disease-response mechanisms to improve the management of complex pathologies, including stroke. On top of that, the simultaneous combination of drugs is also expected to overcome toxicity and dose-associated side effects by lowering the effective dose of each individual compound [14, 15].
In this study, we aimed to use a systems biology–based approach based on artificial intelligence and pattern recognition tools to integrate available biological, pharmacological, and medical knowledge into mathematical models to simulate in silico the complexity of the stroke [16]. By screening these stroke-mimicking models, we have identified a drug combination formed by ceruletide and alpha-1 antitrypsin (CA), two already Food and Drug Administration (FDA)–approved drugs, with theoretical synergic neuroprotective effects. Following our idea of maximizing the number of patients that could benefit from this therapeutic strategy, we have experimentally validated in a mouse stroke model the efficacy of this drug combination in protecting the ischemic brain when administered acutely after stroke induction. Moreover, by using both in vivo and in vitro approaches, we have proved that this drug combination can be safely administered together with t-PA, which supports the future translation of these findings into clinical trials. Last, we also provided evidence that the neuroprotective effects of ceruletide and alpha-1 antitrypsin are partly driven by the modulation of peripheral mediators in circulation.
Material and Methods
In Silico Stroke Modeling, Repositioning, and Identification of CA MoA
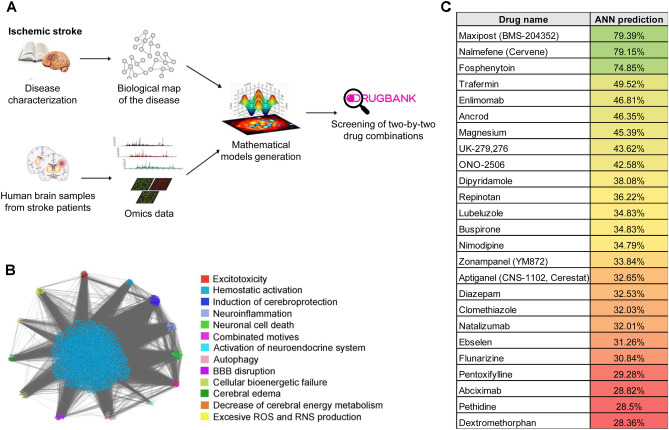
Therapeutic Performance Mapping System (TPMS) technology, a top-down systems biology approach (Anaxomics S.L., Spain), was applied, as previously described [16–18], and validated [19–21] (Supplementary methods). This technology starts off from bibliography and database-based characterization of drugs and conditions [18, 22], and contextualizes them in the human protein network by the use of publicly available protein–protein functional interaction information. The network is then converted to a dynamic model through its training with known pathophysiological relationships (i.e., drug-indication and drug-adverse reactions relationships), and two types of models are built (Fig. 1A): Artificial Neuronal Networks (ANN) [17], with predictive capabilities, and sampling methods [18], with descriptive capabilities. In the current study, we defined 13 relevant processes for ischemic stroke through literature revision, 387 functionally related proteins were assigned to each of them (Supplementary table S1) and this molecular definition was embedded on the human protein network (Fig. 1B). TPMS-based models were constructed from that network, and a combination of both modeling strategies was applied for drug repositioning over DrugBank database [23], using as a benchmark the value obtained for drugs proved unsuccessful to treat stroke so far (Fig. 1C) [24, 25]. This strategy led to CA combination identification, and sampling methods–based models were used for in silico identification of the molecular mechanisms modulated by the combination for further experimental validation (Supplementary methods, Supplementary table S2).
Fig. 1.
In silico mathematical models of ischemic stroke. A Schematic representation of the experimental design. B Snapshot of the full protein network modelled for ischemic stroke, visualized through the Cytoscape software platform. C Artificial Neural Network (ANN) predictive value of treatments already tested in clinical studies of ischemic stroke that have failed to show neuroprotective effect in patients. Gradient of colors (from red to green) indicates increasing % of ANN predictive value
Drugs
Ceruletide (caerulein, Sigma-Aldrich, Germany) and alpha-1 antitrypsin (Prolastin-C, Grifols S.A, Spain) were used in the experiments. The initial dose of each drug was chosen according to an extensive literature-based research [26–39]. Ceruletide was given at a dose of 0.1 mg/kg dissolved in sterile double-distilled water (ddH2O), and alpha-1 antitrypsin at a dose of 60 mg/kg or 480 mg/kg, resuspended in its commercial solution for injection (solvent) and prepared according to manufacturer’s instructions. Sterile ddH2O and Prolastin-C solvent were used as vehicles. Drugs and vehicles were intravenously administered through the retro-orbital sinus (safety study and transient MCAO stroke model) or tail vein (thromboembolic stroke model). Drugs were always administered sequentially.
Animals
All animal procedures were conducted either in compliance with the Spanish or French legislation and following the European Communities Council guidelines (2010/63/EU). Moreover, they were approved either by the Ethics Committee of the Vall d’Hebron Institute of Research (protocol number 74/16) or by the institutional review board (French ministry of Research and by the local ethical committee of Normandy (CENOMEXA)). All experiments were conducted in a randomized manner and in adherence to the ARRIVE guidelines [40]. Sample sizes were not predetermined since preliminary data was not available. C57BL/6 J male mice were used in the experiments (8–12-week-old; Janvier Labs, France), kept in a climate-controlled environment on a 12-h light/12-h dark cycle with food and water available ad libitum. All efforts were made to minimize the possible suffering, pain, or discomfort of the animals. Anesthesia (isoflurane, 5% for induction; 2% for maintenance in medical air, or 70%/30% mixture of NO2/O2) was given to mice via facemask during all surgical procedures described below. Rectal temperature was controlled at 37 ± 0.5 °C throughout all the surgical procedures using a feedback-regulated heating system.
A total of 135 animals were used for the study. Of these, 24 were excluded after applying the following criteria: inappropriate occlusion or reperfusion of the middle cerebral artery (n = 11); massive surgical bleedings (n = 5); death during the surgical procedure (n = 5) or premature death within 24 h after ischemia (n = 3).
Transient Middle Cerebral Artery Occlusion (MCAO) Model
Transient ischemia in the territory of the right middle cerebral artery (MCA) was induced by introducing an intraluminal filament through the external carotid artery, as described elsewhere [41]. In brief, the regional cerebral blood flow (CBF) was monitored close to the region irrigated by the MCA during the entire surgical procedure by affixing a laser Doppler probe (Moor Instruments, UK) to the skull. After surgical exposure of the right bifurcation of the external carotid artery and internal carotid artery, a silicone-coated nylon monofilament (Doccol Corporation, USA; reference number: 602256PK10Re) was introduced to occlude the MCA. The incision was closed with a silk suture and the animal was allowed to recover from anesthesia. The filament was left in place for 90 min. Afterward, mice were re-anesthetized and the filament was gently pulled out to induce reperfusion of the MCA. Only animals that exhibited a reduction of 80% of CBF after filament introduction and a recovery of 80% after filament removal were included in the study. Ten minutes after MCA occlusion, the drugs or vehicles were administered intravenously via the retro-orbital sinus in a blinded manner.
Neuroscore
An investigator blinded to the treatments evaluated all mice and scored them on a composite neurological scale, adapted from previous studies [42, 43]. In brief, neurological score ranges from 0 (healthy) to 39 and represents the sum of the general deficits (0–13): hair [0–2], ears [0–2], eyes [0–3], posture [0–3], spontaneous activity [0–3]; and focal deficits (0–26): body symmetry [0–2], gait [0–4], climbing on a surface held at 45° [0–3], circling behavior [0–3], front limb symmetry [0–4], compulsory circling [0–3], whiskers response to a light touch [0–4], and gripping of the forepaws [0–3]. All animals were evaluated 80 min after MCAO induction (post-occlusion) and 24 h after ischemia.
Sample Collection and Infarct Size Measurement
Under deep terminal anesthesia (5% isoflurane), blood samples were drawn through cardiac puncture and collected in EDTA tubes. Plasma was promptly obtained by centrifugation (3000 g, 10 min, 4 °C) and kept at − 80 °C until further use. Then, mice were transcardially perfused with 20 mL of heparinized cold saline; the brain was immediately collected and cut into 6 serial 1 mm coronal sections to assess infarct volume using 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich) staining, as previously described [44]. TTC images were captured using a CanoScan 4200F (Canon, Japan) and infarct volume was measured using ImageJ software by an investigator blinded to the treatments. Infarct volumes were calculated by integration of the lesion areas as previously described [45]. Results were finally adjusted for edema by dividing the measured value by the scaling factor I/C (I: area of the ipsilateral hemisphere; C: area of the contralateral hemisphere), and expressed in cubic millimeters (mm3). After scanning, brains were immediately frozen at − 80 °C until further use.
Thromboembolic Model
The distal occlusion of the right MCA by thrombin injection and t-PA-induced reperfusion was conducted as previously described [46]. In brief, before the surgical procedure, a pipette was made with hematologic micropipettes (calibrated at 15 mm/µL; Assistent ref. 555/5; Hoecht, Sondheim-Rhoen, Germany) by using an electrophysiology puller (PC-10; Narishige) and pneumatically filled with purified murine alpha-thrombin (0.05 mg; Stago BNL). Mice were placed in a stereotaxic device and the right MCA was exposed after a small craniotomy and the excision of the dura mater. The pipette was introduced into the lumen of the MCA and 1 µL of purified murine alpha-thrombin (1UI) was injected to induce the formation of a clot in situ. The efficacy of the occlusion was assessed using laser Doppler flowmetry, with the optic fiber placed above the MCA territory before and up to 10 min after MCA occlusion (MCAo).
Ten minutes after thrombin injection, a catheter was inserted into the tail vein to allow the intravenous administration of the drugs or vehicle in a blinded manner. Ten minutes later (20 after thrombin injection), 200 µL of human recombinant t-PA (10 mg/kg, Actilyse®, Boeringher Ingelheim) was injected (10% bolus, 90% perfusion for 40 min). The control group received the same volume of saline under identical conditions.
Grip Strength
The grip strength test (BIOSEB, France) was used to assess neuromuscular functions in mice by determining the strength displayed by the animal relative to the forepaw-grasping reflex. Five tests per mouse were performed to reduce variabilities, with 1 min resting time in between to obtain the mean strength. Measurements were registered in grams the day before MCAO surgery (baseline acquisition) and at 24 h, 48 h, and 72 h of ischemia and expressed as % of baseline values to normalize individual with its baseline values (= 100%).
Infarct Size Measurement
Neuroimages were acquired by magnetic resonance imaging 24 h after ischemia. Experiments were carried out on a Pharmascan 7 T/12 cm system using surface coils (Bruker, Germany). T2-weighted images were acquired using a multi-slice multi-echo sequence (TE/TR 33/2500 ms with 70 × 70 × 500 µm3 spatial resolution) and used for lesion size as quantification using the ImageJ software. T2*-weighted sequences were used to monitor if animals underwent hemorrhagic events (TE/TR 7.7/500 ms with 70 × 70 × 500 µm3 spatial resolution). Two-dimensional time-of-flight angiographies (TE/TR 12/7 ms) were acquired to analyze the MCA angiogram and identify undesired spontaneous recanalization (data not shown).
Blood Biochemical Parameters
Routine clinical biochemistry parameters including urea, creatinine, creatine kinase (CK), aspartate transaminase (AST), and alanine transaminase (ALT) were analyzed and quantitatively measured in the clinical laboratories from Hospital Vall d’Hebron (Barcelona, Spain).
Western Blot
Frozen TTC-stained ischemic and contralateral hemispheres from each animal were homogenized with freshly prepared ice-cold lysis buffer (1% phosphatase inhibitor cocktail 3 (Sigma-Aldrich), 0.1 M PMSF (Sigma-Aldrich), 0.5% Aprotinin (Sigma-Aldrich) diluted in ready-to-use RIPA buffer (Sigma-Aldrich)). One gram of tissue per sample was homogenized in 1 mL of lysis buffer with a homogenizer drill (5 mm diameter bit). Then, the tissue was centrifuged (15.294 g, 12 min, 4 °C) and supernatants were stored at − 80 °C until further use. The total protein concentration of the homogenates was measured by the BCA method (PierceTM BCA Protein Assay, ThermoFisher, USA). Equal protein amounts (30–40 µg) were resolved in a 5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis under reducing conditions and transferred to nitrocellulose or polyvinylidene difluoride (PVDF) membrane. Membranes were blocked with 10% non-fat milk and incubated with the following primary antibodies: ANGPT1 (Proteintech, USA; 1/500), EGFR (Biorbyt, UK; 1/1000), β-actin (Sigma-Aldrich, 1/5000), or β-tubulin (Abcam, 1/2000). Membranes were then incubated with the secondary antibody linked to horseradish peroxidase (HRP) (Sigma-Aldrich) (anti-rabbit HRP for ANGPT1 and EGFR, 1/2000; or anti-mouse HRP for β-actin and β-tubulin, 1/2000). The substrate reaction was developed with chemiluminescent reagent Luminol (ThermoFisher) and analyzed with Odyssey Li-Cor. Western blots were quantified using ImageJ free software. Positive signals were corrected by β-actin or β-tubulin signal, used as a loading control. Afterward, each ipsilateral hemisphere (IP) band signal was normalized by the respective contralateral hemisphere (CL) band signal to obtain signal ratios.
Olink® Proteomic Array
The Target 96 Mouse Exploratory panel was performed by Olink® Proteomics (Uppsala, Sweden) to simultaneously measure 92 proteins that encompass a broad range of biological functions and pathways (Supplementary table S3). Thirty microliters of frozen brain lysates from the IP and CL hemispheres from both experimental groups (n = 6/group) was analyzed with the Olink® Proteomics technology in accordance with the established protocols and policy. Data is provided as normalized protein expression (NPX) values, on log2 scale Olink Proteomics’ arbitrary units. The quality of each sample was assessed by evaluating the deviation from the median value of the controls (pooled plasma samples) for each individual sample, which was less than 0.3 NPX from the median. Intra-assay coefficient of variance was 4% (reference intra CV < 15%).
ELISA Immunoassay
A sandwich ELISA array was assayed to determine the levels of EGFR and ANGPT1 in mouse plasma, using commercially available ELISA kits (EGFR: Cat. #ab201275, Abcam, UK, and ANGPT1: Cat.#EK1296, Boster, USA). Optical densities (OD) were measured by duplicate in a SynergyTM Mx microplate reader (BioTek Instruments Inc., USA). Samples displaying a CV higher than 20% were excluded from the analysis.
In Vitro Clot Formation and Lysis Assay
A pool of plasma was obtained from 10 healthy volunteers from the ISSYS cohort [47] (5 men and 5 women, age 78 [75.25–82.25], with no medical records of coagulation disorders). In brief, blood samples were collected in citrate tubes and centrifuged at 1500 g, 15 min at 4 °C. All 10 plasma samples were mixed together for 2 h at 4 °C and then kept at − 80 °C until further use.
The assay was conducted in a microtiter 96-well plate with 25 µL of citrate plasma added to 75 µL of assay buffer (0.05 M Tris–HCl (Sigma-Aldrich), 1 M NaCl (Sigma-Aldrich), 1.245 ng (83 ng/mL) of t-PA (Actilyse®) at pH = 7.4). A final volume of 50µL of activation mix (7.5 mM CaCl2 and 0.03U/mL thrombin) was also added to start the clot reaction. OD was immediately read at 405 nm and every 40 s for 40-min total assay time at 37 °C using a BIO-TEK Elx-808 microplate reader and Gen5 software. Internal controls without t-PA were also run in each independent experiment. Three independent experiments were performed for each experimental condition and all samples were run per triplicate. Only those with a CV < 20% were used for the final analysis.
Ceruletide and alpha-1 antitrypsin were loaded at two different doses: low dose (CA, 83 ng/mL and 498 ng/mL, respectively) and high dose, 10 times higher than the low dose (CA 10x, 830 ng/mL and 4980 ng/mL, respectively). To determine the in vitro low doses, we sought to accurately reproduce the in vivo physiological ratio between the circulating concentration of t-PA and drugs in the in vitro environment. For that purpose, we first extrapolated the concentration of ceruletide and alpha-1 antitrypsin in circulation immediately after its administration, calculated based on a standard mouse of 25 g (8–12 weeks) and 80 mg/kg of blood content [48]. We then calculated the in vitro dose of ceruletide and alpha-1 antitrypsin based on the ratio between the in vivo (10 mg/kg, 125 µg/mL) and in vitro (0.083 µg/mL) t-PA concentration [49], following the formula: [drug in vitro] = [drug in vivo] × [tPA in vitro] / [tPA in vivo].
Drugs were also tested at two different time-points: before starting clot formation (t0) and once the clot was already formed (t1, 14.78 ± 4.5 min from starting point), when reactions reached their maximum OD.
Statistical Analysis
GraphPad Prism 6.0 was used for analyzing data and creating graphs. Results are given as mean ± SD (normally distributed variables) or median ± interquartile range (non-normal-distributed variables). Data Gaussian distribution was checked using the Shapiro–Wilk and Kolmogorov–Smirnov tests. Comparisons among independent experimental groups were performed through unpaired Student’s t-tests or one-way ANOVA followed by Tukey’s multiple comparison test for normal-distributed variables, and the Mann–Whitney test or a Kruskall-Wallis test, followed by Dunn’s multiple comparison test for non-normal-distributed variables. Data comparison over time within each experimental group was performed by paired Student’s t-test (normal variables) or Wilcoxon test (non-normal variables). A p-value < 0.05 was considered to be statistically significant in all cases.
The analysis of the proteomic data was performed with R-studio software (R-studio, Boston, USA). Differentially expressed proteins were selected based on a linear model analysis for paired samples, implemented in the Bioconductor limma package [50]. Statistically significant proteins were considered when False Discovery Rate (FDR) adjusted p-value < 0.10. Enrichment analysis on differentially expressed proteins between experimental groups was performed using the EnrichR web-based tool through the Fisher exact test [51]. Logarithmic base 10 transformation of the p-value was computed to obtain p-value ranking.
Results
The Combination of Ceruletide and Alpha-1 Antitrypsin Presents Synergic Neuroprotective Potential In Silico
To discover potential neuroprotective drug combinations to successfully treat ischemic stroke, we initially applied a machine-learning approach to simulate and computationally reproduce the stroke pathophysiology (Fig. 1A). In brief, we generated systems biology–based artificial maps for stroke modeling that covered 13 well-known pathological pathways of stroke extracted from literature review (Supplementary Table S1), which encompassed a total of 6640 proteins (Fig. 1B). These maps were then computationally converted into mathematical stroke models and fed with biological knowledge available related to stroke (see Supplementary methods, Supplementary Table S2). To further enrich these bibliography-based stroke models, we also incorporated valuable datasets from 4 different proteomics and transcriptomics studies previously conducted in our lab on human brain samples from patients who died due to ischemic stroke [52–54].
Once the mathematical simulation of stroke was created, disease-orientated drug repositioning neuroprotective solutions were then acquired by perturbing these virtual stroke models with already approved drugs from the DrugBank Database (v4.3) [23]. First, to validate and demonstrate the accuracy of the in silico generated models of ischemic stroke, we screened 25 drugs that have been previously tested as neuroprotectants in clinical stroke trials (Fig. 1C). Since none of them has been approved for therapeutic use during clinical stroke management [24, 25], used the ANN predictive values obtained in our mathematical models to establish a threshold for the optimal identification of the new candidates in this study.
We next screened the virtual stroke models with more than 5 million two-by-two combinations of drugs to identify potential neuroprotective drug combinations that surpassed that threshold. Furthermore, we only considered combinations with synergistic effect (ANN predictive value of the combination > 20% of the individual drug values). Additionally, and in order to further identify those with high translational potential, we also applied several specific filter criteria to the screening (see Supplementary methods), including approval status for the individual drugs; individual drugs could not have previous knowledge of association to hypotension or hemorrhages; and all individual drugs could not present incompatibilities for intravenous administration (aiming to widen the therapeutic management to more patients within the hyper-acute phase of stroke).
From all drug combinations that fulfilled the established criteria, we selected the combination formed by ceruletide and alpha-1 antitrypsin to be further studied in the in vivo stroke models. For the selection of CA among all candidate combinations, different criteria were taken into consideration. Twenty-five drug combinations with the highest ANN predictive value (ranging from 88 to 96%) that fulfilled all established criteria were pre-selected. Of those, 16 drug combinations containing monoclonal antibodies were initially discarded due to higher difficulties in their administration and translation into clinics. Six out of the 9 remaining combinations presented the highest ANN predictive value (92%), including CA. Final selection among these candidates was performed based on literature information, their market status, and the ANN predictive value of each individual drug compared to that of the combination. Regarding CA, the ANN of A1AT alone was 40%, of ceruletide 51%, whereas the ANN value of the combination raised up to 92%, showing as well a high value of the predicted synergic effect (81%). Moreover, pieces of evidence in the literature gave us information about the possible protective role of both proteins, since A1AT showed an anti-inflammatory [28, 55, 56] and an anti-apoptotic role [35], while ceruletide may modulate neurotoxicity [57, 58], and also some studies supported the neuroprotective potential of the individual drugs [26, 59].
Ceruletide and Alpha-1 Antitrypsin in Combination Showed Neuroprotection in a Mouse Model of Stroke
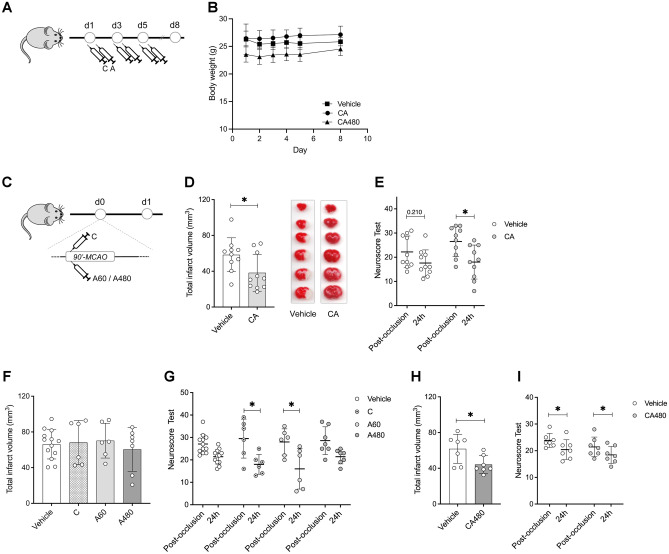
To further explore the predicted synergic effect of ceruletide and alpha-1 antitrypsin, we aimed to experimentally test the neuroprotective effect of the drug combination in a mouse model of cerebral ischemia. To prevent any toxic effect derived from the pre-selected doses and the co-administration of the two drugs at the same time, we first investigated whether the administration of both drugs in combination was safe. To that end, we initially evaluated safety outcomes on naïve animals receiving three alternate-day doses of the drug combination (CA) or the respective drug vehicles (Fig. 2A). Clinical blood biochemical parameters measured 8 days after the first drug dose showed similar levels between treatment groups (Supplemental table S2). Moreover, animals receiving either CA or vehicle treatment did not present significant body weight loss (Fig. 2B), overall suggesting no apparent side effects from these drugs when given in combination.
Fig. 2.
Administration of CA showed neuroprotection after stroke. A Experimental design of the safety study. B Body weight monitoring of animals treated with vehicle or ceruletide 0.1 mg/kg and alpha-1 antitrypsin at a dose of 60 mg/kg (CA60) or 480 mg/kg (CA480). C Experimental design of the efficacy study. D Infarct volumes (mm3) of animals treated with vehicle (n = 10) or ceruletide (0.1 mg/kg) + alpha-1 antitrypsin (60 mg/kg) (CA, n = 10) 24 h after cerebral ischemia. E Neurological deficits of animals evaluated post-occlusion (80 min after MCAO induction) and 24 h after the ischemic event. F Infarct volumes (mm3) of animals treated with vehicle (n = 10) or single drugs (n = 6 for ceruletide (C), n = 6 for alpha-1 antitrypsin at a dose of 60 mg/kg (A60) and n = 7 for alpha-1 antitrypsin at 480 mg/kg (A480)). G Neurological deficits of animals evaluated post-occlusion and 24 h after the ischemic event. H Infarct volumes (mm3) of animals treated with vehicle (n = 10) or ceruletide (0.1 mg/kg) + alpha-1 antitrypsin (480 mg/kg) (CA480, n = 10) 24 h after cerebral ischemia. I Neurological deficits of animals evaluated post-occlusion and 24 h after the ischemic event. In all cases, mean ± SD is shown. * indicates p < 0.05 and **p < 0.01
After considering the administration of the drug combination safe, we next intended to validate its theoretical neuroprotective effects in vivo (Fig. 2C). To that end, both drugs from the combination were given sequentially to mice immediately after MCAO. Our results show that the administration of CA exhibited neuroprotection 24 h after stroke by reducing 39.42% the infarct volume compared to the vehicle group (p = 0.022, Fig. 2D). In addition, only CA-treated mice improved their neurological scale scoring over time, when comparing post-occlusion and 24 h deficits (p = 0.012, Fig. 2E).
We also demonstrated the synergic effects of CA in front of single-drug treatment (Fig. 2F), since none of the drugs reduced the infarct volume when given alone compared to the vehicle group (p = 0.956 for ceruletide and p = 0.888 for alpha-1 antitrypsin). However, both individual treatments showed an improvement in the neurological outcome 24 h after ischemia compared to post-occlusion deficits (p = 0.005 for ceruletide and p = 0.003 for alpha-1 antitrypsin, Fig. 2G).
The Synergic Neuroprotective Effects of CA Persist Regardless of the Alpha-1 Antitrypsin Dose
To evaluate whether the observed therapeutic effects were dose-dependent, we tested the neuroprotective effect of the drug combination after increasing the dose of alpha-1 antitrypsin up to 480 mg/kg, while maintaining the dose of ceruletide at 0.1 mg/kg. This criterion was established based on previous literature supporting the use of higher doses of alpha-1 antitrypsin in humans, but not of ceruletide [60, 61].
In terms of safety, and consistent with the lower dose results, animals receiving the higher dose of alpha-1 antitrypsin in combination with ceruletide (CA480) did not suffer any body weight loss. Also, all biochemical blood parameters showed similar levels between vehicle- and CA480-treated animals (Fig. 2B, Supplementary Table S4).
Efficacy results were also similar to those reported for the lower dose of alpha-1 antitrypsin: mice treated with the CA480 treatment showed a significant infarct volume reduction compared to the vehicle treatment (p = 0.031, Fig. 2H), whereas the single treatment with alpha-1 antitrypsin at a high dose did not show infarct reduction (p = 0.913, Fig. 2F). The neurological outcome improved over time for both experimental groups similarly (p = 0.011 for CA480, p = 0.010 for vehicle, Fig. 2I), whereas this improvement was not observed in animals treated only with alpha-1 antitrypsin at a high dose (p = 0.162, Fig. 2G). Based on these results, further experiments to study the beneficial effects of the CA treatment were conducted with ceruletide (0.1 mg/kg) and alpha-1 antitrypsin at the lower dose (60 mg/kg).
CA Does Not Impair the Thrombolytic Activity of t-PA
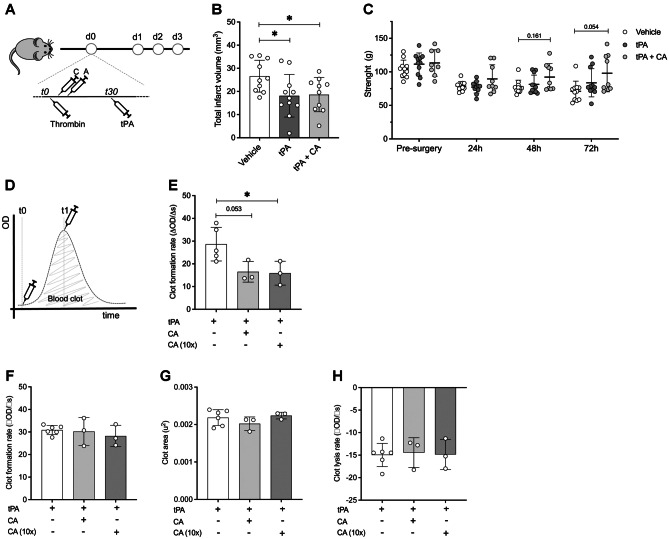
To test the translational potential of our CA treatment in the context of stroke thrombolysis, we made use of a thromboembolic stroke model in mice with t-PA-induced reperfusion (Fig. 3A). Infarct volume was here evaluated through magnetic resonance imaging 24 h after stroke onset and functional deficits were recorded by means of the grip strength test daily within 3 days after stroke. Animals receiving t-PA alone or together with CA showed a substantial reduction of the ischemic lesion when compared to the non-thrombolyzed vehicle group (p = 0.025 for t-PA; p = 0.033 for t-PA + CA; Fig. 3B). However, no differences were observed between t-PA-treated animals, either alone or together with CA (p = 0.896). None of the animals receiving t-PA alone or together with CA presented possible signs of hemorrhagic transformation, an event that was only observable in one animal from the non-thrombolyzed vehicle group. Interestingly, the administration of CA before thrombolysis also showed a trend towards an improvement in the forepaw strength 72 h after stroke compared to the vehicle-treated group (p = 0.054), which was unexpectedly not observed in t-PA-treated mice. These results suggest a plausible effect of the drug combination on improving the functional outcome after stroke beyond the hyper-acute phase of the disease (Fig. 3C).
Fig. 3.
CA does not impair the thrombolytic activity of t-PA. A Experimental design. Drugs were given intravenously at a dose of 0.1 mg/kg for ceruletide and 60 mg/kg for alpha-1 antitrypsin. B Infarct volumes (mm3) of animals treated with vehicle (vehicle, n = 10), t-PA (n = 11), and t-PA together with ceruletide + alpha-1 antitrypsin (tPA + CA, n = 9) 24 h after cerebral ischemia. C Grip strength test measurements performed before MCAO and 24, 48, and 72 h after surgery. Histograms represent strength of both forepaws. Data was assessed in grams. D Representation of the clot formation and lysis experiment showing the two time-points of drug administration: t0 and t1. E Clot formation rate when drugs were added at t0. F Clot formation rate when drugs were added at t1. G Clot area and H clot lysis rate parameters of the experiments performed when drugs were added at t1. In all cases, mean ± SD is shown. * indicates p < 0.05 and #p < 0.1
To further explore whether the drug combination can be safely administered together with t-PA in humans, we made use of an in vitro clot/lysis assay approach, which simulated the formation of a blood clot and its lysis by means of the protease activity of t-PA [49]. When CA was added to the clot/lysis assay at baseline (t0, Fig. 3D), we observed a significant reduction in the rate of clot formation at both, the CA therapeutic dose and overdosed (10 times higher), compared to the vehicle-treated group (Fig. 3E). This result pointed at a possible effect of CA in the prevention of blood clotting. To specifically evaluate the effect of the neuroprotective therapy on the t-PA-related clot lysis, CA was added to the assay after the clot was formed (t1, Fig. 3D). This way, equal clot formation rates were ensured before any treatment was given (Fig. 3F). However, no differences were observed in the total area under the clot formation and lysis curve (Fig. 3G), neither in the clot lysis rate (Fig. 3H), indicating no alterations of CA on the human t-PA clot-lysis profile in vitro.
Evaluation of the Synergic Mechanism of Action of the CA
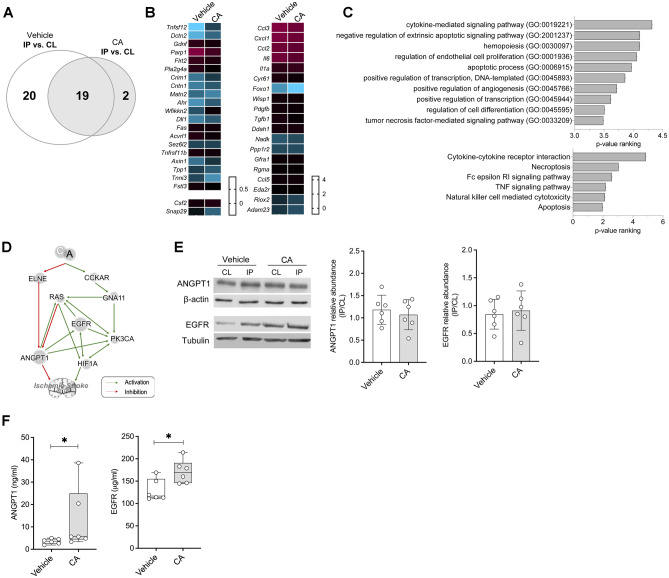
To elucidate a mechanistic background for our neuroprotective findings, we initially performed proteomics on the ipsilateral and contralateral tissue of CA- and vehicle-treated mice (Fig. 2A). Out of the 92 explored proteins, 39 of them were differentially expressed (DE) between the IP and the CL brain hemispheres of vehicle-treated mice, but only 21 between brain hemispheres from animals treated with CA (Fig. 4A, B, Supplementary table S5). Interestingly, 19 of these proteins were DE in both experimental groups, suggesting a minor effect of the treatment on these concrete molecules. In support of this hypothesis, we also found that the fold changes of these 19 DE proteins did not statistically differ between experimental groups (Fig. 4B).
Fig. 4.
Identification and validation of the synergic mechanisms of action of CA. A Venn’s diagram of the differentially expressed proteins between the IP and CL brain hemispheres of vehicle- and CA-treated mice evaluated in the Olink proteomic array. B Heat maps of the differentially expressed proteins between the IP and CL brain hemispheres of mice treated with vehicle (left) or CA (right). C Biological significance analysis of the proteins modulated by CA. D Predicted molecular mechanism of action of CA on ischemic stroke obtained from the in silico generated mathematical models of stroke. E Representative Western blot images and quantification of brain levels of ANGPT1 and EGFR in vehicle- and CA-treated animals (n = 5/group). The ratio between IP and CL levels within each animal is depicted. F Quantification of ANGPT1 and EGFR levels in mouse plasma samples (n = 7/group). In all cases, mean ± SD is shown. * indicates p < 0.05 and **p < 0.01. Abbreviations: ANGPT1, angiopoetin-1; CCKAR, cholecystokinin receptor type A; CL, healthy contralateral brain hemisphere; EGFR, epidermal growth factor receptor; ELNE, neutrophil elastase; GNA11, G protein subunit alpha 11; HIF1A; hypoxia-inducible factor 1-alpha; IP, ipsilateral brain hemisphere; PK3CA, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha
To further unravel the effect of the drug combination in the ischemic brain, we next focused our attention on the proteins that had been either up- or down-regulated by the CA treatment, compared to the vehicle-treated group. By analyzing these proteins for overrepresentation of biological functions, we found that our treatment exerted effects by modulating cytokine-mediated pro-inflammatory signaling pathways, necrotic and apoptotic processes, as well as by promoting endothelial cell proliferation and angiogenesis (Fig. 4C).
To further gain insight into the mechanism underlying the synergic neuroprotective effects of ceruletide and alpha-1 antitrypsin, we next made use once more of the in silico mathematical simulation of ischemic stroke. This time, by applying sampling methods strategies, models draw up the predicted mechanisms of action that could explain the beneficial effects of CA (Fig. 4D). Interestingly, modulated mechanisms involved the Endothelial Grown Factor Receptor (EGFR) and angiopoietin 1 (ANGPT1), among other more ubiquitous proteins, such as the Ras GTPase, PIK3CA (Phosphatidylinositol-4,5-Bisphosphate 3-Kinase Catalytic Subunit Alpha), and the hypoxia-inducible factor 1-alpha (HIF-1a) (Fig. 4D). Since it is already known that ANGPT1 has a crucial role in stroke pathophysiology [62, 63] and due to the proposed implication of EGFR in promoting cell proliferation and survival [64], we further investigate these two proteins in our CA- or vehicle-treated stroke mice. Experimental testing of ANGPT1 and EGFR did not show differences in brain protein expression when comparing treated and non-treated animals (Fig. 4E). On the contrary, circulating plasma EGFR and ANGPT1 levels were found elevated in animals treated with CA, compared to the vehicle group (Fig. 4F). However, no differences were observed in animals treated with either agent alone (Supplementary Figure S1), reinforcing the synergistic effect of the drug combination. Altogether, these results support the notion that the proposed CA treatment might lead to a synergic neuroprotective effect by modulating EGFR and ANGPT1 systemically.
Discussion
During the past decades, a large number of studies have devoted their efforts to identify potential neuroprotective strategies to treat ischemic stroke, most of which were directed at modulating single biological motives involved in the pathophysiology of the disease. However, the failure of many of these drugs in experimental and clinical studies has become conceptual proof that perhaps the individual modulation of single pathological mechanisms might not be sufficient for attenuating the progression of a highly complex and multifactorial disease such as stroke [5]. Thus, the simultaneous targeting of several pathways of the ischemic cascade with a combination of neuroprotective agents could offer a better therapeutic approach for stroke. In fact, this strategy has already proven its high efficacy in other complex pathologies, including in neurodegenerative [65, 66] and neoplastic [67–69] diseases: to treat amyotrophic lateral sclerosis, a drug cocktail consisting of an inhibitor of microglial activation (Minocycline), a glutamate antagonist (Riluzone), and a calcium-channel antagonist (Nimodipine) has already shown neuroprotective effects in vivo compared to the minimal effect described for the single treatments [66]. Similarly, to treat early-stage breast cancer, a drug cocktail formed by a DNA-intercalating agent (Anthracycline), an immunosuppressor (Cyclophosphamide) and an antimetastatic drug (Taxane), is being already used in clinical practice [67]. These attractive results reinforce the idea that the synergic action of drugs targeting different pathways might be required to successfully treat complex and multifactorial diseases such as stroke, but no combinational stroke treatment has been yet studied in clinical trials so far [70].
With this concept in mind, we here aimed at discovering potential combinational treatments for stroke by means of a systems biology and drug repositioning approach. Our objective was focused on finding a therapy that could benefit the largest number of patients, and therefore it should be given as soon as possible after stroke onset, even before the stroke diagnosis is confirmed in the medical center, to maximize its effectiveness [4]. Systems biology has shown to be in our hands a powerful method to accurately reproduce the whole process of stroke. Indeed, all known biological processes of the pathophysiology of stroke were precisely depicted in our stroke models, including among others neuronal cell death, brain-blood barrier disruption, ROS and RNS production, and neuroinflammation. Also, our in silico simulation of stroke incorporated valuable data from proteomics and transcriptomic studies conducted on brain necropsies from ischemic stroke patients. These datasets provided substantial unbiased raw information of the main molecules that change in the brain following an ischemic stroke at both the protein and the gene levels, which overall improved the performance of the mathematical models used in the present investigation.
To guarantee the therapeutic success of the outcoming drug combinations, we initially tested in our newly generated mathematical models of stroke the performance of 25 drugs that have previously failed their translation into clinics. These include, among others, agents targeting the glutamate-induced excitotoxicity, such as Aptiganel [71] or Lubeluzole [72]; the acute inflammatory response and the infiltration of immune cells into the damaged brain, including UK-279,276 [73], Natalizumab [74], and Enlimomab [75], or the overload of cellular calcium, for instance through Nimodipine [76] and Flunarizine [77]. The screening of these drugs allowed us to set a threshold that all the newly identified combinations of drugs had to overcome. This criterion made more likely the success when experimentally testing in pre-clinical models and even in future clinical trials any combination of drugs resulting from this screening approach.
Alpha-1 antitrypsin is a well-known serine protease inhibitor with described anti-inflammatory and anti-apoptotic properties [78]. Less is known about the therapeutic qualities of ceruletide, which was initially described as a neuromodulator of the dopaminergic system [79] and has been also involved in the reduction of glutamate-induced neuronal death [57, 80]. Our findings suggest that the neuroprotective effect of the CA treatment could be attributed to an enhanced synergic interaction of both drugs, rather than an additive action, since we could not demonstrate drugs’ individual therapeutic efficacy. Also, our data do support that the neuroprotective effect of CA is not dependent on alpha-1 antitrypsin dose, since we observed a similar reduction of the infarct volume when mice were treated with either the low or the high dose of alpha-1 antitrypsin in combination with ceruletide. Nevertheless, treatment with alpha-1 antitrypsin alone has previously shown protection against cerebral ischemic injury in other pre-clinical studies [26]. Differences in species, the modeling of stroke, and the drug doses and routes of administration, among others, might be some of the reasons behind the discrepancy.
Our results also suggested that the synergic neuroprotective effects of the drug combination might be triggered through systemic actions. We have demonstrated that the combinational treatment but not the single agents increased ANGPT-1 and EGFR levels in circulation, well-known growth factors that promote angiogenesis [81] and cell proliferation [64], respectively. Of note, the upregulation and activation of these molecules have been already described to induce neuroprotection by reducing the infarct size and the brain-blood barrier leakiness in stroke pre-clinical models [81–84], which could also explain the therapeutic findings of our combinational treatment. Concretely, ANGPT1 helps in the maintenance of the vascular quiescence by inhibiting the increased vascular permeability against inflammatory mediators [85] protecting cell-to-cell contacts and tight junctions of the endothelial cells [86]. Moreover, decreased circulating levels of ANGPT1 within the hyper-acute phase in ischemic stroke patients have been associated with poorer outcomes [63]. Thus, it has been suggested that therapeutic interventions upregulating ANGPT1 could potentially improve stroke outcomes [87], which is in line with our findings. However, no data is available regarding the possible protective effects that increased circulating EGFR levels may exert in the brain; thus, future studies are needed to elucidate its neuroprotective mechanism. Besides these alterations in circulation, we could only identify minor molecular changes in the brain due to the treatment. Enrichment analysis revealed that ceruletide and alpha-1 antitrypsin might be modulating inflammation and cell death processes in the brain, both well-known key mechanisms defining stroke outcome [88, 89]. Besides, processes related to endothelial cell proliferation and angiogenesis are also stimulated by the treatment, perhaps favoring an increased microvessel density in the infarcted region which has been associated with improved stroke outcomes [90]. Altogether, further studies are needed to confirm these initial findings and explore in more detail the molecular mechanisms of the observed therapeutic effect of ceruletide and alpha-1 antitrypsin in combination.
It should be also highlighted that no significant toxic or side effects were found in any of our experiments. In this same regard, we have demonstrated that the drug combination could be safely administered together with t-PA, overall paving the way for a future translation of this encouraging therapeutic strategy to clinical trials in stroke patients. Interestingly, this treatment would particularly benefit most those patients who cannot receive t-PA treatment due to any of the well-known medical contraindications, but it will neither cause any deleterious effect in those who are treated with t-PA at their arrival to the hospital.
The present study also stands for some limitations that should be overcome in future studies. Firstly, the experimental validation of the neuroprotective effects has only been performed in young male mice. Performing studies in different models of cerebral ischemia, including permanent strokes that resemble clinical presentations of large vessel strokes, in animals of different sexes, ages, and comorbidities, as proposed in the STAIR recommendations [91] would accelerate the translation into clinics. In addition, these future studies should also include complementary behavioral and functional assessment approaches to fully evaluate the potential of this treatment for improving stroke outcome and recovery, thus confirming these initial but encouraging findings. Secondly, drug administration time-points here used correlate with a clinical scenario in which therapeutic interventions are performed within the pre-hospital phase of care, such as in the ambulance, or early post-arrival emergency department time period. Delayed administration of the drug combination also needs to be tested to fully corroborate its acute neuroprotective capacity after stroke. Similarly, the long-term impact of such acute treatment, within the sub-acute and chronic phases of the disease, deserves further attention in future pre-clinical and clinical trials. Third, further studies are needed to fully elucidate the specific mechanisms of action of the drug combination. In this regard, exploring transcriptomic and proteomic changes in the brain with new and high-throughput technologies would probably improve the identification of the underlying effects of the combinational treatment in the ischemic brain. In the same line, it would be also interesting to deepen into the systemic alterations triggered by the drug combination, to confirm whether the beneficial action of the combinational treatment is mainly driven by the modulation of stroke-triggered peripheral mediators, beyond a local action in the ischemic brain.
In conclusion, we have here used a new systems biology approach to generate mathematical models that simulate the pathophysiology of ischemic stroke. This in silico representation allowed us to identify a drug combination formed by ceruletide and alpha-1 antitrypsin which showed synergic neuroprotective effects after ischemia in vivo. Overall, our findings shed light on a new powerful strategy for developing future therapies for ischemic stroke.
Supplementary Information
Below is the link to the electronic supplementary material.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author Contribution
A.S and J.M conceived and designed the experiments. R.V, L.A, and T.S generated the in silico mathematical model and performed the drug repositioning experiment. A.S, L.R, and C.O performed all animal experiments. H.R, P.G-R, and A.S conducted the in vitro experimental studies. A.R and J.M supervised the experiments. A.S and L.R drafted the manuscript. All authors have critically reviewed the article content and approved it in its final version.
Funding
Neurovascular Research Laboratory acknowledges funding for this project by PI15/00354 and PI18/00804 grants from Instituto Carlos III; the Spanish neurovascular research network INVICTUS PLUS (RD16/0019/0021) also from the Instituto de Salud Carlos III (co-financed by the European Regional Development Fund, FEDER); the program 2017-SGR-1427 from the Generalitat de Catalunya; and the “Advanced Neuroprotection Repurposing Drugs and Nutraceuticals for Stroke in Andalusia: NARDNIA Project,” and COMBINA2 project both funded by the Andalusian Ministry of Health (PE-0527–2019 and PY20-01351). L.R. is supported by a predoctoral fellowship grant (IFI17/00012), from Instituto de Salud Carlos III.
Availability of Data and Material
Supplemental material for this article is available online.
Declarations
Ethical Approval
All animal procedures were conducted in adherence to the ARRIVE guidelines [40], either in compliance with the Spanish or French legislation and following the European Communities Council guidelines (2010/63/EU). They were approved either by the Ethics Committee of the Vall d’Hebron Institute of Research (protocol number 74/16) or by the institutional review board (French ministry of Research and by the local ethical committee of Normandy (CENOMEXA).
Conflict of Interest
A.S., L.R., and J.M. are co-inventors of a patent covering “Methods and compositions for treating ischaemia in a subject” (Application nº EP19382600.5), co-owned by VHIR and Anaxomics Biotech. Other authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Alba Simats and Laura Ramiro both contributed equally to this work.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fiehler J, Gerloff C. Mechanical thrombectomy in stroke. Dtsch Arztebl Int. 2015;112:830–836. doi: 10.3238/arztebl.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med [Internet]. 2008;359:1317–29. Available from: http://www.nejm.org/doi/abs/10.1056/NEJMoa0804656. [DOI] [PubMed]
- 4.Shkirkova K, Saver JL, Starkman S, Wong G, Weng J, Hamilton S, et al. Frequency, predictors, and outcomes of prehospital and early postarrival neurological deterioration in acute stroke: exploratory analysis of the FAST-MAG randomized clinical trial. JAMA Neurol. 2018. [DOI] [PMC free article] [PubMed]
- 5.Onwuekwe I, Ezeala-Adikaibe B. Ischemic stroke and neuroprotection. Ann Med Health Sci Res. 2012. [DOI] [PMC free article] [PubMed]
- 6.O’Collins VE, Macleod MR, Donnan GA, Horky LL, Van Der Worp BH, Howells DW. 1,026 Experimental treatments in acute stroke. Ann Neurol. 2006. [DOI] [PubMed]
- 7.Moretti A, Ferrari F, Villa RF. Neuroprotection for ischaemic stroke: current status and challenges. Pharmacol Ther. Elsevier B.V. 2015;146:23–34. [DOI] [PubMed]
- 8.Goenka L, Uppugunduri Satyanarayana CR, S SK, George M. Neuroprotective agents in acute ischemic stroke—a reality check. Biomed Pharmacother. 2019. [DOI] [PubMed]
- 9.Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol. Springer Berlin Heidelberg. 2017;133:245–61. [DOI] [PMC free article] [PubMed]
- 10.Pujol A, Mosca R, Farrés J, Aloy P. Unveiling the role of network and systems biology in drug discovery. Trends Pharmacol Sci. 2010;31:115–123. doi: 10.1016/j.tips.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Mei H, Feng G, Zhu J, Lin S, Qiu Y, Wang Y, et al. A practical guide for exploring opportunities of repurposing drugs for CNS diseases in systems biology. Methods Mol Biol. 2016;1303:531–547. doi: 10.1007/978-1-4939-2627-5_33. [DOI] [PubMed] [Google Scholar]
- 12.Casas AI, Hassan AA, Larsen SJ, Gomez-Rangel V, Elbatreek M, Kleikers PWM, et al. From single drug targets to synergistic network pharmacology in ischemic stroke. Proc Natl Acad Sci USA. 2019. [DOI] [PMC free article] [PubMed]
- 13.Langhauser F, Casas AI, Dao VTV, Guney E, Menche J, Geuss E, et al. A diseasome cluster-based drug repurposing of soluble guanylate cyclase activators from smooth muscle relaxation to direct neuroprotection. npj Syst Biol Appl. 2018. [DOI] [PMC free article] [PubMed]
- 14.Jaeger S, Igea A, Arroyo R, Alcalde V, Canovas B, Orozco M, et al. Quantification of pathway cross-talk reveals novel synergistic drug combinations for breast cancer. Cancer Res. 2017;77:459–469. doi: 10.1158/0008-5472.CAN-16-0097. [DOI] [PubMed] [Google Scholar]
- 15.Lehar J, Krueger A, Avery W, Heilbut A, Johansen L. Synergistic drug combinations improve therapeutic selectivity. Nat Biotechnol. 2009;27:659–666. doi: 10.1038/nbt.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mas JM, Pujol A, Aloy P, Farrés J. Methods and systems for identifying molecules or processes of biological interest by using knowledge discovery in biological data. 2010.
- 17.Artigas L, Coma M, Matos-Filipe P, Aguirre-Plans J, Farrés J, Valls R, et al. In-silico drug repurposing study predicts the combination of pirfenidone and melatonin as a promising candidate therapy to reduce SARS-CoV-2 infection progression and respiratory distress caused by cytokine storm. PLoS One. 2020. [DOI] [PMC free article] [PubMed]
- 18.Jorba G, Aguirre-Plans J, Junet V, Segú-Vergés C, Ruiz JL, Pujol A, et al. In-silico simulated prototype-patients using TPMS technology to study a potential adverse effect of sacubitril and valsartan. PLoS One. 2020. [DOI] [PMC free article] [PubMed]
- 19.Romeo-Guitart D, Forés J, Herrando-Grabulosa M, Valls R, Leiva-Rodríguez T, Galea E, et al. Neuroprotective drug for nerve trauma revealed using artificial intelligence. Sci Rep. 2017;Submitted:1–15. [DOI] [PMC free article] [PubMed]
- 20.Iborra-Egea O, Santiago-Vacas E, Yurista SR, Lupón J, Packer M, Heymans S, et al. Unraveling the molecular mechanism of action of empagliflozin in heart failure with reduced ejection fraction with or without diabetes. JACC Basic to Transl Sci. 2019. [DOI] [PMC free article] [PubMed]
- 21.Lorén V, Garcia-Jaraquemada A, Naves JE, Carmona X, Mañosa M, Aransay AM, et al. Anp32e, a protein involved in steroid-refractoriness in ulcerative colitis, identified by a systems biology approach. J Crohn’s Colitis. 2019. [DOI] [PubMed]
- 22.Segú-Vergés C, Coma M, Kessel C, Smeets S, Foell D, Aldea A. Application of systems biology-based in silico tools to optimize treatment strategy identification in Still’s disease. Arthritis Res Ther. 2021. [DOI] [PMC free article] [PubMed]
- 23.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. Oxford University Press; 2018;46:D1074–82. [DOI] [PMC free article] [PubMed]
- 24.Chamorro Á, Dirnagl U, Urra X, Planas AM. Neuroprotection in acute stroke: targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016. [DOI] [PubMed]
- 25.Ginsberg MD. Current status of neuroprotection for cerebral ischemia synoptic overview. Stroke. 2009;40:111–115. doi: 10.1161/STROKEAHA.108.528877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moldthan HL, Hirko AC, Thinschmidt JS, Grant MB, Li Z, Peris J, et al. Alpha 1-antitrypsin therapy mitigated ischemic stroke damage in rats. J Stroke Cerebrovasc Dis. 2014. [DOI] [PMC free article] [PubMed]
- 27.Toldo S, Mauro AG, Marchetti C, Rose SW, Mezzaroma E, Van Tassell BW, et al. Recombinant human alpha-1 antitrypsin-Fc fusion protein reduces mouse myocardial inflammatory injury after ischemia-reperfusion independent of elastase inhibition. J Cardiovasc Pharmacol. 2016. [DOI] [PubMed]
- 28.Gold M, Dolga AM, Koepke J, Mengel D, Culmsee C, Dodel R, et al. α1-antitrypsin modulates microglial-mediated neuroinflammation and protects microglial cells from amyloid-β-induced toxicity. J Neuroinflammation. 2014;11:1–11. doi: 10.1186/s12974-014-0165-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols DP, Jiang D, Happoldt C, Berman R, Chu HW. Therapeutic effects of α1-antitrypsin on psedumonas aeruginosa infection in ENaC transgenic mice. PLoS One. 2015. [DOI] [PMC free article] [PubMed]
- 30.Jedicke N, Struever N, Aggrawal N, Welte T, Manns MP, Malek NP, et al. Alpha-1-antitrypsin inhibits acute liver failure in mice. Hepatology. 2014. [DOI] [PubMed]
- 31.Moreno JA, Ortega-Gomez A, Rubio-Navarro A, Louedec L, Ho-Tin-Noé B, Caligiuri G, et al. High-density lipoproteins potentiate α1-antitrypsin therapy in elastase-induced pulmonary emphysema. Am J Respir Cell Mol Biol. 2014. [DOI] [PubMed]
- 32.Collins CB, Aherne CM, Ehrentraut SF, Gerich ME, McNamee EN, McManus MC, et al. Alpha-1-antitrypsin therapy ameliorates acute colitis and chronic murine ileitis. Inflamm Bowel Dis. 2013. [DOI] [PMC free article] [PubMed]
- 33.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. α1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A. 2005. [DOI] [PMC free article] [PubMed]
- 34.Pott GB, Scott Beard K, Bryan CL, Merrick DT, Shapiro L. Alpha-1 antitrypsin reduces severity of Pseudomonas pneumonia in mice and inhibits epithelial barrier disruption and Pseudomonas invasion of respiratory epithelial cells. Front Public Heal. 2013. [DOI] [PMC free article] [PubMed]
- 35.Toldo S, Seropian IM, Mezzaroma E, Van Tassell BW, Salloum FN, Lewis EC, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2011. [DOI] [PubMed]
- 36.Heindl M, Tuennemann J, Sommerer I, Mössner J, Hoffmeister A. Loss of Bace1 in mice does not alter the severity of caerulein induced pancreatitis. PLoS One. 2015. [DOI] [PMC free article] [PubMed]
- 37.Daemen MARC, Heemskerk VH, Van’t Veer C, Denecker G, Wolfs TGAM, Vandenabeele P, et al. Functional protection by acute phase proteins α1-acid glycoprotein and α1-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation. 2000. [DOI] [PubMed]
- 38.Woods SE, Leonard MR, Hayden JA, Brophy MB, Bernert KR, Lavoie B, et al. Impaired cholecystokinin-induced gallbladder emptying incriminated in spontaneous “black” pigment gallstone formation in germfree Swiss Webster mice. Am J Physiol - Gastrointest Liver Physiol. 2015. [DOI] [PMC free article] [PubMed]
- 39.Gaw AJ, Hills DM, Spraggs CF. Characterization of the receptors and mechanisms involved in the cardiovascular actions of sCCK‐8 in the pithed rat. Br J Pharmacol. 1995. [DOI] [PMC free article] [PubMed]
- 40.Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLOS Biol. 2013;4:35–44. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clark W, Lessov N, Dixon M, Eckenstein F. Monofilament intraluminal middle cerebral artery occlusion in the mouse. Neurol Res. 1997;19:641–648. doi: 10.1080/01616412.1997.11740874. [DOI] [PubMed] [Google Scholar]
- 42.Orsini F, Villa P, Zangari R, Zanier ER, Gesuete R, Stravalaci M, et al. Targeting mannose binding lectin confers long lasting protection with a surprisingly wide therapeutic window in cerebral ischemia. Circulation. 2012;126:1484–1494. doi: 10.1161/CIRCULATIONAHA.112.103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Simoni MG, Storini C, Barba M, Catapano L, Arabia AM, Rossi E, et al. Neuroprotection by complement (C1) inhibitor in mouse transient brain ischemia. J Cereb Blood Flow Metab. 2003;23:232–239. doi: 10.1097/01.WCB.0000046146.31247.A1. [DOI] [PubMed] [Google Scholar]
- 44.Bederson JB, Pitts LH, Germano SM, Nishimura MC, Davis RL, Bartkowski HM. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke. 1986;17:1304–1308. doi: 10.1161/01.STR.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 45.Morancho A, García-Bonilla L, Barceló V, Giralt D, Campos-Martorell M, Garcia S, et al. A new method for focal transient cerebral ischaemia by distal compression of the middle cerebral artery. Neuropathol Appl Neurobiol. 2012;38:617–627. doi: 10.1111/j.1365-2990.2012.01252.x. [DOI] [PubMed] [Google Scholar]
- 46.Orset C, Macrez R, Young AR, Panthou D, Angles-Cano E, Maubert E, et al. Mouse model of in situ thromboembolic stroke and reperfusion. Stroke. 2007. [DOI] [PubMed]
- 47.Riba-Llena I, Jarca CI, Mundet X, Tovar JL, Orfila F, López-Rueda A, et al. Investigating silent strokes in hypertensives: a magnetic resonance imaging study (ISSYS): rationale and protocol design. BMC Neurol [Internet]. 2013;13:130–7. Available from: http://bmcneurol.biomedcentral.com/articles/10.1186/1471-2377-13-130. [DOI] [PMC free article] [PubMed]
- 48.Riches AC, Sharp JG, Thomas DB, Smith SV. Blood volume determination in the mouse. J Physiol. 1973. [DOI] [PMC free article] [PubMed]
- 49.Carter AM, Cymbalista CM, Spector TD, Grant PJ. Heritability of clot formation, morphology, and lysis: the EuroCLOT study. Arterioscler Thromb Vasc Biol. 2007;27:2783–2789. doi: 10.1161/ATVBAHA.107.153221. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015. [DOI] [PMC free article] [PubMed]
- 51.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles G V., et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013. [DOI] [PMC free article] [PubMed]
- 52.Cuadrado E, Rosell A, Colomé N, Hernández-Guillamon M, García-Berrocoso T, Ribo M, et al. The proteome of human brain after ischemic stroke. J Neuropathol Exp Neurol [Internet]. 2010;69:1105–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20940630. [DOI] [PubMed]
- 53.García-Berrocoso T, Llombart V, Colàs-Campàs L, Hainard A, Licker V, Penalba A, et al. Single cell immuno-laser microdissection coupled to label-free proteomics to reveal the proteotypes of human brain cells after ischemia. Mol Cell Proteomics. 2018;17:175–189. doi: 10.1074/mcp.RA117.000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramiro L, García-Berrocoso T, Briansó F, Goicoechea L, Simats A, Llombart V, et al. Integrative multi-omics analysis to characterize human brain ischemia. Mol Neurobiol. 2021. [DOI] [PubMed]
- 55.Pott GB, Chan ED, Dinarello CA, Shapiro L. α-1-Antitrypsin is an endogenous inhibitor of proinflammatory cytokine production in whole blood. J Leukoc Biol. 2009;85:886–895. doi: 10.1189/jlb.0208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janciauskiene S, Larsson S, Larsson P, Virtala R, Jansson L, Stevens T. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by α1-antitrypsin. Biochem Biophys Res Commun. 2004. [DOI] [PubMed]
- 57.Katsuura G, Shinohara S, Shintaku H, Eigyo M, Matsushita A. Protective effect of CCK-8 and ceruletide on glutamate-induced neuronal cell death in rat neuron cultures: possible involvement of CCK-B receptors. Neurosci Lett. 1991. [DOI] [PubMed]
- 58.Xu C, Xue D, Su MC, Du LF, Gu X, Yang CL, et al. The influence of proglumide, a putative CCK antagonist, on cerebral ischemia in gerbil. Peptides. 1987. [DOI] [PubMed]
- 59.Eigyo M, Katsuura G, Shintaku H, Shinohara S, Katoh A, Shiomi T, et al. Systematic administration of a cholecystokinin analogue, ceruletide, protects against ischemia-induced neurodegeneration in gerbils. Eur J Pharmacol. 1992. [DOI] [PubMed]
- 60.Seyama K, Nukiwa T, Sato T, Suzuki M, Konno S, Takahashi K, et al. Safety and pharmacokinetics of Alpha-1 MP (Prolastin ® -C) in Japanese patients with alpha 1 -antitrypsin (AAT) deficiency. Respir Investig. 2019. [DOI] [PubMed]
- 61.Sargent EN, Boswell W, Hubsher J. Cholecystokinetic cholecystography: efficacy and tolerance studies of ceruletide. Am J Roentgenol. 1978. [DOI] [PubMed]
- 62.Sun J, Yu L, Huang S, Lai X, Milner R, Li L. Vascular expression of angiopoietin1, α5β1 integrin and tight junction proteins is tightly regulated during vascular remodeling in the post-ischemic brain. Neuroscience. 2017. [DOI] [PubMed]
- 63.Golledge J, Clancy P, Maguire J, Lincz L, Koblar S, Mcevoy M, et al. Plasma angiopoietin-1 is lower after ischemic stroke and associated with major disability but not stroke incidence. Stroke. 2014. [DOI] [PubMed]
- 64.Wee P, Wang Z. Epidermal growth factor receptor cell proliferation signaling pathways. Cancers (Basel). 2017. [DOI] [PMC free article] [PubMed]
- 65.Rosenfeld J. Multi-drug therapy in amyotrophic lateral sclerosis: the case for a multi-drug approach. Muscle Nerve. 2004. [DOI] [PubMed]
- 66.Kriz J, Gowing G, Julien JP. Efficient three-drug cocktail for disease induced by mutant superoxide dismutase. Ann Neurol. 2003. [DOI] [PubMed]
- 67.Fujii T, Le Du F, Xiao L, Kogawa T, Barcenas CH, Alvarez RH, et al. Effectiveness of an adjuvant chemotherapy regimen for early-stage breast cancer. JAMA Oncol. 2015. [DOI] [PMC free article] [PubMed]
- 68.Dai W, Wang X, Song G, Liu T, He B, Zhang H, et al. Combination antitumor therapy with targeted dual-nanomedicines. Adv Drug Deliv Rev. 2017. [DOI] [PubMed]
- 69.Elmaci İ, Altinoz MA. A metabolic inhibitory cocktail for grave cancers: metformin, pioglitazone and lithium combination in treatment of pancreatic cancer and glioblastoma multiforme. Biochem Genet. 2016. [DOI] [PubMed]
- 70.Du Y, Zhang X, Ji H, Liu H, Li S, Li L. Probucol and atorvastatin in combination protect rat brains in MCAO model: upregulating Peroxiredoxin2, Foxo3a and Nrf2 expression. Neurosci Lett Elsevier Ireland Ltd. 2012;509:110–115. doi: 10.1016/j.neulet.2011.12.054. [DOI] [PubMed] [Google Scholar]
- 71.Albers GW, Goldstein LB, Hall D, Lesko LM. Aptiganel hydrochloride in acute ischemic stroke a randomized controlled trial Gregory. JAMA. 2001;286:2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 72.Diener HC. Multinational randomised controlled trial of lubeluzole in acute ischaemic stroke. European and Australian Lubeluzole Ischaemic Stroke Study Group. Cerebrovasc Dis. 1998;8:172–81. [DOI] [PubMed]
- 73.Krams M, Lees KR, Hacke W, Grieve AP, Orgogozo J-M, Ford GA, et al. Acute Stroke Therapy by Inhibition of Neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34:2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 74.Elkins J, Veltkamp R, Montaner J, Johnston SC, Singhal AB, Becker K, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol [Internet]. Elsevier Ltd; 2017;16:217–26. Available from: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed]
- 75.Acute E, Trial S. Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology [Internet]. 2001;57:1428–34. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11673584. [DOI] [PubMed]
- 76.Kaste M, Fogelholm R, Erilä T, Palomäki H, Murros K, Rissanen A, et al. A randomized, double-blind, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke. 1994;25:1348–1353. doi: 10.1161/01.STR.25.7.1348. [DOI] [PubMed] [Google Scholar]
- 77.Franke CL, Palm R, Dalby M, Schoonderwaldt HC, Hantson L, Eriksson B, et al. Flunarizine in stroke treatment (FIST): a double-blind, placebo-controlled trial in Scandinavia and the Netherlands. Acta Neurol Scand. 1996;93:56–60. doi: 10.1111/j.1600-0404.1996.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 78.Carrell RW. α1-antitrypsin: molecular pathology, leukocytes, and tissue damage. J Clin Invest. 1986;78:1427–1431. doi: 10.1172/JCI112731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Albus M, Anckenheil M, Münch U, Naber D. Ceruletide: a new drug for the treatment of schizophrenic patients? Arch Gen Psychiatry. 1984;41:528. doi: 10.1001/archpsyc.1984.01790160114018. [DOI] [PubMed] [Google Scholar]
- 80.Shinohara S, Katsuura G, Eigyo M, Shintaku H, Ibii N, Matsushita A. Inhibitory effect of CCK-8 and ceruletide on glutamate-induced rises in intracellular free calcium concentrations in rat neuron cultures. Brain Res. 1992. [DOI] [PubMed]
- 81.Moxon J V., Trollope AF, Dewdney B, de Hollander C, Nastasi DR, Maguire JM, et al. The effect of angiopoietin-1 upregulation on the outcome of acute ischaemic stroke in rodent models: a meta-analysis. J. Cereb. Blood Flow Metab. 2019. [DOI] [PMC free article] [PubMed]
- 82.Chen M, Wu S, Shen B, Fan Q, Zhang R, Zhou Y, et al. Activation of the δ opioid receptor relieves cerebral ischemic injury in rats via EGFR transactivation. Life Sci. 2021. [DOI] [PubMed]
- 83.Peng DH, Liu YY, Chen W, Hu HN, Luo Y. Epidermal growth factor alleviates cerebral ischemia-induced brain injury by regulating expression of neutrophil gelatinase-associated lipocalin. Biochem Biophys Res Commun. 2020. [DOI] [PubMed]
- 84.Moss A. The angiopoietin: TIe 2 interaction: a potential target for future therapies in human vascular disease. Cytokine Growth Factor Rev. 2013. [DOI] [PubMed]
- 85.Valable S, Montaner J, Bellail A, Berezowski V, Brillault J, Cecchelli R, et al. VEGF-induced BBB permeability is associated with an MMP-9 activity increase in cerebral ischemia: both effects decreased by Ang-1. J Cereb Blood Flow Metab. 2005. [DOI] [PubMed]
- 86.Siddiqui MR, Mayanil CS, Kim KS, Tomita T. Angiopoietin-1 regulates brain endothelial permeability through PTPN-2 mediated tyrosine dephosphorylation of occludin. PLoS One. 2015. [DOI] [PMC free article] [PubMed]
- 87.Zheng Q, Zhu D, Bai Y, Wu Y, Jia J, Hu Y. Exercise improves recovery after ischemic brain injury by inducing the expression of angiopoietin-1 and Tie-2 in rats. Tohoku J Exp Med. 2011. [DOI] [PubMed]
- 88.Zhang SR, Phan TG, Sobey CG. Targeting the immune system for ischemic stroke. Trends Pharmacol. Sci. 2021. [DOI] [PubMed]
- 89.Simats A, García-Berrocoso T, Montaner J. Neuroinflammatory biomarkers: from stroke diagnosis and prognosis to therapy. Biochim Biophys Acta - Mol Basis Dis [Internet]. Elsevier B.V.; 2016;1862:411–24. Available from: http://dx.doi.org/10.1016/j.bbadis.2015.10.025. [DOI] [PubMed]
- 90.Hatakeyama M, Ninomiya I, Kanazawa M. Angiogenesis and neuronal remodeling after ischemic stroke. Neural Regen. Res. 2020. [DOI] [PMC free article] [PubMed]
- 91.Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke treatment academic industry roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 2011;42:2645–2650. doi: 10.1161/STROKEAHA.111.618850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Supplemental material for this article is available online.