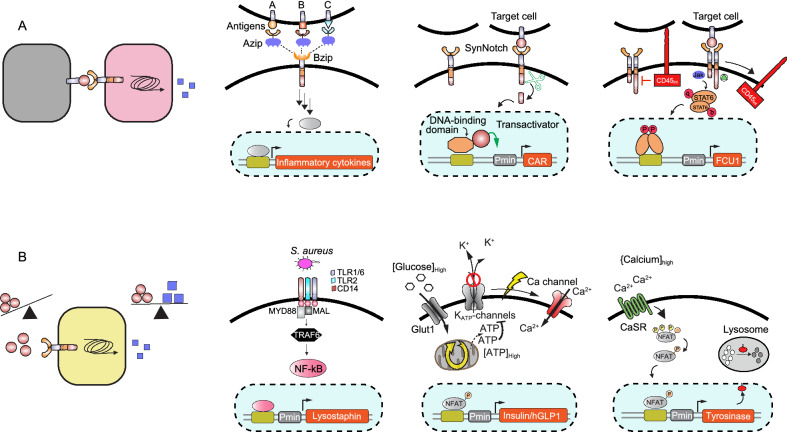
Figure 2.
Closed-loop-mediated cell-based therapies. (A) Cell-to-cell mediated control of designer cells. This approach utilizes an antigen (biomarker) exposed on the surface of the targeted cell, and a physical interaction is needed for activation of the designer cell. SUPRA is a generalized CAR platform that enables a unique T cell to bind to different user-defined antigens and to trigger an endogenous signaling pathway upon activation. SynNotch relies on cleavage of a chimeric receptor after cell-cell interaction. The cleaved intracellular domain of synNotch translocates to the nucleus and initiates transcription of a desired therapeutic gene. Another cell-contact sensor is based on physical segregation of CD43ex-45int. In the absence of a target cell, CD43ex-45int suppresses an implemented JAK/STAT pathway. Once the designer cell binds to a targeted cell, CD43ex-45int is segregated from the cell–cell interface due to the physical force applied to the large extracellular domain of CD43ex-45int. Therefore, the JAK/STAT pathway will be activated and initiate expression of a therapeutic protein. (B) Metabolite-mediated closed-loop systems. A high level of soluble biomarker can stimulate designer cells to produce therapeutic agents to balance the level of the biomarker. In an immunomimetic cell, a TLR platform is used to activate the NFκB pathway and express an antibacterial peptide (lysostaphin) to treat MRSA. The β-cell-mimetic designer cell is designed to sense a high level of glucose in a diabetic model and to produce insulinogenic proteins (insulin and hGLP1) through expression of Cav1.3 on HEK293 cells rewired to a synthetic expression unit. The biomedical tattoo utilizes a designer cell that enables early disease detection by sensing a high level of calcium, and produces tyrosinase upon activation. Tyrosinase mediates production of melanin, a black pigment, in the engineered designer cells