Abstract
Cerebellum is a key structure for functional motor recovery after stroke. Enhancing the cerebello-motor pathway by paired associative stimulation (PAS) might improve upper limb function. Here, we conducted a randomized, double-blind, sham-controlled pilot trial investigating the efficacy of a 5-day treatment of cerebello-motor PAS coupled with physiotherapy for promoting upper limb motor function compared to sham stimulation. The secondary objectives were to determine in the active treated group (i) whether improvement of upper limb motor function was associated with changes in corticospinal excitability or changes in functional activity in the primary motor cortex and (ii) whether improvements were correlated to the structural integrity of the input and output pathways. To that purpose, hand dexterity and maximal grip strength were assessed along with TMS recordings and multimodal magnetic resonance imaging, before the first treatment, immediately after the last one and a month later. Twenty-seven patients were analyzed. Cerebello-motor PAS was effective compared to sham in improving hand dexterity (p: 0.04) but not grip strength. This improvement was associated with increased activation in the ipsilesional primary motor cortex (p: 0.04). Moreover, the inter-individual variability in clinical improvement was partly explained by the structural integrity of the afferent (p: 0.06) and efferent pathways (p: 0.02) engaged in this paired associative stimulation (i.e., cortico-spinal and dentato-thalamo-cortical tracts). In conclusion, cerebello-motor-paired associative stimulation combined with physiotherapy might be a promising approach to enhance upper limb motor function after stroke.
Clinical Trial Registration URL: http://www.clinicaltrials.gov. Unique identifier: NCT 02284087.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13311-022-01205-y.
Keywords: Stroke, Cerebellum, Transcranial magnetic stimulation, Diffusion tensor imaging, Functional magnetic resonance imaging
Introduction
Non-invasive brain stimulation techniques are promising tools when combined with physical training to improve motor function after stroke [1, 2]. However, up to date, targeting the primary motor cortex (M1) has given contradictory results, probably because (i) the heterogeneity of lesions and/or the small sample sizes [3] and (ii) the stimulation mainly focused on the damaged corticospinal tract. Alternative targeted strategies may involve undamaged pathways playing a key role in motor recovery [4]. Among these candidates, the cerebellum might be a target of choice [4, 5] given that it is involved in sensory motor integration and motor skill learning [6].
Although several studies have demonstrated decreased functional interaction between the cerebellum and the motor cortex after stroke [7–9], few have investigated the contribution of this dysfunction in motor deficits of the upper limb. A study combining diffusion imaging (DTI) and functional MRI (fMRI) found that reduced cerebello-cortical connectivity was independently associated with greater deficits of the hand, even when considering the damage to the corticospinal tract [10]. Moreover, on a structural level, the integrity of the dentato-thalamo-cortical tract, which connects the cerebellum to M1, has been associated with residual general motor output in chronic stroke [11]. Together, these findings suggest that cerebellum may be a promising target for clinical trials.
Paired associative stimulation (PAS) is a plasticity induction protocol thought to induce spike-time-dependent plasticity changes. Based on a classical conditioning-test TMS paradigm, PAS can strengthen or weaken the synaptic transmission between two nodes, for example, between an afferent and an efferent pathway [12–14]. Indeed, in healthy subjects, repetitive pairing of a conditioning stimulus applied over the cerebellum and a test stimulus over M1 can induce long-term potentiation/depression-like plastic changes [15] in M1 as described in [14]. So far, cerebello-motor (CER_M1) PAS has never been explored in stroke patients despite the promising role of the cerebellum in motor stroke recovery.
Here, we conducted a randomized, sham-controlled, double-blind pilot study with parallel groups receiving either 5 consecutive days of active or sham CER_M1 PAS immediately followed by physical training in stroke with motor deficits of the upper limb.
The predefined primary objective was to assess the efficacy of CER_M1 PAS to promote upper limb recovery. We hypothesized that active CER_M1 PAS would improve upper limb motor function and particularly hand dexterity, to a greater extent than sham CER_M1 PAS. The first secondary objective was to determine whether improvement in upper limb motor function was associated with changes in corticospinal excitability or changes in functional M1 activity, with the assumption that the proportion of activation in the ipsilesional M1 will be increased in patients who benefited the most from the intervention. Finally, the last secondary objective was to determine whether CER_M1 PAS-induced changes correlated to the integrity of the afferent (i.e., dentato-thalamo-cortical tract) and the efferent (i.e., corticospinal tract) pathways as assessed before the intervention. We hypothesized that clinical effects observed in the active CER_M1 PAS group would depend on the structural integrity of the cerebello-motor network as assessed by diffusion MRI.
Methods
Study Design
The study consisted of a prospective, randomized, sham-controlled, parallel groups design, double-blinded trial in stroke patients. Eligible patients who met the inclusion criteria were prospectively and consecutively screened in the stroke unit of the Pitié-Salpêtrière Hospital. Patients were then referred to the Brain Institute of the Pitié-Salpêtrière Hospital where the protocol took place.
The trial investigated whether 5 days of CER_M1 PAS was effective in improving upper limb motor function after stroke. Patients were fully assessed with clinical scores, electrophysiological TMS recordings, and MRI examinations before, immediately after the last treatment and a month later (Supplemental Material Fig. 1).
For recruitment reasons, the study initially scheduled for 2 years was extended to 3 years. There were no changes in the selection criteria or study design during the recruitment period. The trial was terminated when the recruitment period was over.
Population
Inclusion criteria were (1) ischemic stroke as confirmed by MRI, (2) stroke onset > 30 days prior to inclusion, (3) Fugl-Meyer score < 60 for the motor upper limb domain after the removal of the reflex evaluation item as in [16], and (4) a spared precentral “hand knob” area as confirmed by MRI to ensure that M1, which was the node between afferent and efferent pathways engaged in the CER_M1 PAS protocol, was not damaged.
Exclusion criteria consisted of (1) homonymous hemianopia, given that an alteration of the visual field could have impacted the visuo-motor task used for the fMRI paradigm, (2) complete paralysis of the hand muscles (Medical Research Council muscle power scale ≤ 1, i.e., no voluntary active movement), (3) age < 18 or > 85 years, (4) contraindications to MRI or TMS [17], and (5) severe cognitive deficits that hampered the ability of the patients to understand and follow the protocol according to the physician in charge of the patient.
This study was conducted according to established good clinical practice guidelines and was approved by the local ethical committee (CPP Ile de France VI #85–14). Written informed consent from each participant or from a legal proxy/family member was obtained.
Randomization and Treatment Protocol
Randomization and Masking
Each participant was randomly assigned in a 1:1 ratio to active or sham CER_M1 PAS. A computer-generated randomization was achieved using a secure website (http://randoweb.aphp.fr), which is based on an algorithm that used a minimal sufficient balancing method to prevent imbalances in the three predefined following variables: age, initial Fugl-Meyer score, and side of the lesion. Double blinding was ensured by the intervention of three investigators: (i) one unblinded investigator performed the randomization and applied treatment sessions; (ii) two other blinded investigators were responsible for clinical, electrophysiological, and neuroimaging assessments. Patients were also blinded to the treatment allocation.
The estimation of the sample size was calculated using a F-test (repeated measures ANOVA, 3 measurements, with within-between interaction) with the G*power software (http://www.gpower.hhu.de/en.html). Type I error was set at 5% and power at 80%. To demonstrate an effect size of 0.25, 14 patients per group were needed.
Treatment Protocol
CER_M1 PAS interventions were delivered each day of the week for a total of 5 sessions, immediately followed by 45 min of physical training by a physiotherapist focusing on upper limb functions.
The active group received 120 pairs of stimuli at 0.2 Hz delivered by two MAGSTIM 2002 stimulator units (Magstim, Dyfed, UK). The conditioning stimulus was delivered through a 110-mm double-cone coil over the contralesional cerebellum. The center of the coil was moved right- or leftwards off the midpoint by 3 cm along a line between the inion and the mastoid process [14]. The test stimulus was delivered using a 70 mm figure-of-eight-shaped coil centered over the ipsilesional M1. The coil was first held tangential to the scalp over the presumed hand knob area as determined by the anatomical 3D reconstruction of each participant’s brain (Brainsight2, Rogue Research, Inc., Montreal, Canada). The optimal coil position (“hotspot”) was then determined as the site where TMS consistently produced the largest motor-evoked potentials (MEPs) in the contralateral first dorsal interosseus muscle at a suprathreshold intensity. When MEPs could not be elicited in the ipsilesional hemisphere, the hotspot was defined anatomically in the hand knob area. Resting Motor Threshold was measured using the relative frequency method. Surface EMG was recorded from the first dorsal interosseus muscle of both hands at rest.
The intensity of the conditioning stimulus was set at 90% of the resting motor threshold (rMT) and at 140% rMT for the test stimulus. The low intensity of the conditioning stimulus was chosen to minimize confounding effects due to direct brainstem stimulation [18] and maintained under 50% of the maximal stimulator output to avoid pain. For patients in whom MEP could not be elicited, the intensity was set at 100% for the ipsilesional M1 and 50% of the maximal stimulator output for the contralesional cerebellar target. The interstimulus interval was set at 2 ms [14]. In the sham group, the test stimulus was similar and delivered by the same coil as described above (70 mm figure-of-eight-shaped coil). A sham coil was applied over the cerebellum. This sham coil produces a sound mimicking the one produced by the active coil.
After each session, discomfort was rated with a Likert-like scale from − 3 (very uncomfortable) to 3 (very positive sensations).
Physical therapy sessions lasted around 45 min and were delivered immediately after the intervention. All patients were able to activate some muscles voluntarily in the affected upper limb, but only some of them demonstrated functional finger and wrist movements. Different types of exercises were delivered according to the patients’ potential: active-assisted range of motion exercises combined with motor imagery, strength training against gravity, and task specific training (manipulating spoon, glass of water, bottle, buttoning).
Multimodal Assessments
All assessments were performed before the first CER_M1 PAS intervention (baseline, D0), immediately after the last intervention (D5) and a month later (D30).
Clinical Assessments
Upper limb motor performance was evaluated with the Fugl-Meyer score at inclusion. This score allows us to counterbalance the clinical severity of the two groups (see randomization). As the cerebellum plays a major role in motor function and coordination, we chose to evaluate the Jebsen-Taylor hand function test (JTT). As in [19], we included only six of the seven JTT subtests: turning cards, picking up small objects, picking up beans with a teaspoon, stacking checkers, and lifting light and heavy cans. For both hands, each subtest was performed three times and averaged. The total JTT time was computed by adding the average duration of all subtests. It is important to note that no learning or fatigue effect was detected using a repeated measure ANOVA (F(2.228): 0.16, p: 0.85).
In addition, we measured the grip strength (GS) of the affected and unaffected hands three times for both hands and averaged the trials (MIE, Medical Research Ltd., (http://www.mie-uk.com/pgripmyo/index.html)).
This allowed us to assess the specificity of the CER_M1 PAS as we expected an improvement in goal-directed tasks more than grip force because of the implication of cerebellum in motor coordination and motor control.
TMS Assessment
The TMS evaluation was performed to provide MEPs amplitude in the first dorsal interosseus muscles. Detailed description of the procedure is provided in Supplemental Material. The peak-to-peak MEP amplitude was recorded at 140% rMT from both hemispheres. For patients in whom a MEP cannot be elicited in the ipsilesional side, MEP amplitude was imputed at 0 mV as in [20].
MRI Assessment
Whole brain MRI was performed at 3 T (Magnetom VERIO, Siemens, Erlangen, Germany) with a 32-channel head coil. The MRI protocol included (i) 3D T1-weighted MPRAGE images, (ii) a fluid-attenuated inverse recovery (FLAIR) sequence, (iii) a multiband spin-echo echo-planar high-angular resolution diffusion imaging sequence, and (iv) a fMRI acquired during a motor task. Infarct volumes were delineated on the FLAIR images by a trained neurologist in order to calculate the lesion volume (FSLview). Acquisition parameters can be found in the Supplemental Material.
Functional MRI was performed to record activations in the primary motor cortices during a visuomotor task described in Supplemental Material [21].
The functional images were processed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm). Briefly, before pre-processing, images of patients with right-sided lesions were flipped so the lesion was in the left hemisphere. A general linear model analysis was performed at the subject level using box-car conditions convoluted with the canonical hemodynamic response function in order to model average activation during all trials. Head movement parameters were included as nuisance variables. Activity in the motor cortex was determined by contrasting hand movement blocks vs. rest using a statistical threshold of T = 2.34 (p < 0.01, uncorrected) within the precentral gyrus regions of interest from the AAL atlas (Montreal Neurological Institute space).
Diffusion MRI with probabilistic tractography in a common space (see Supplemental Material) was performed to reconstruct bilateral corticospinal and the dentato-thalamo-cortical tracts. Mean weighted fractional anisotropy (FA) values were obtained for the whole ipsilesional and contralesional corticospinal and dentato-thalamo-cortical tracts.
Data and Statistical Analysis
Because of the inter-individual variability due to the severity of the motor impairment at inclusion (D0), for clinical, electrophysiological and DTI data, we computed a ratio (r) by dividing the respective value obtained on the affected hemisphere/hand by the one of the unaffected hemisphere/hand (JTTr, GSr, MEPr, and FAratio) [20]. Before this step, we verified the stability of the clinical data of the non-affected side by a mixed model ANOVA.
Statistical analysis was performed with per-protocol analysis approach. The analysis was performed by a blinded investigator using MedCalc software (version 12.5.0, Belgium, 2013). Kolmogorov-Smirnoff tests were used to assess normality of the data. Descriptive statistics consisted of mean and standard deviations (SD). Comparisons of proportions were determined by a chi-squared test and the quantitative variables were compared by t-tests.
Primary Objective
A mixed model ANOVA was used to test the effects of CER_PAS M1 on the JTTr and GSr. The within subject effect was TIME (D0, D5, D30), and the between-subject effect was GROUP (active or sham CER_M1 PAS). Post-hoc comparisons were performed using independent t-tests between groups corrected for multiple comparisons by a Bonferroni. Sphericity was checked with Mauchly’s test, using the Greenhouse–Geisser correction when necessary.
Secondary Objectives
The secondary objectives were to determine which changes occurred in the motor network in patients who benefited the most from the active CER_M1 PAS treatment. Pearsons’ correlation coefficients were used with 95% confidence interval (95%CI) between clinical improvement after CER_M1 PAS and changes in the motor network. Clinical improvement was measured by the difference between JTTr (or GSr) after CER_M1 PAS vs. JTTr (or GSr) at D0 (ΔJTTr, ΔGSr). Changes in the motor network were defined as the difference in corticospinal excitability measured by MEPr at D5/D30 vs. MEPr at D0 (ΔMEPr_D5, ΔMEPr_D30) or the changes in the ipsilesional M1 activation during a motor task fMRI paradigm between D5 or D30 and D0 (ΔM1_D5, ΔM1_D30). Thus, a negative difference value of ΔJTTr means that the patient clinically improved and vice versa. By contrast, a positive Δ value of GSr, MEPr, or M1 activity means that hand strength or corticospinal excitability or functional activations improved, respectively, parameters.
Finally, to determine which characteristics at baseline (D0) could explain changes in the JTTr (i.e., the patients who are more likely to benefit from the active intervention), we computed correlations with pre-specified candidates, selected to reflect structural integrity of the cerebello-motor pathway, i.e., FAratio in the corticospinal and dentate-thalamo-cortical tracts.
Results
Population
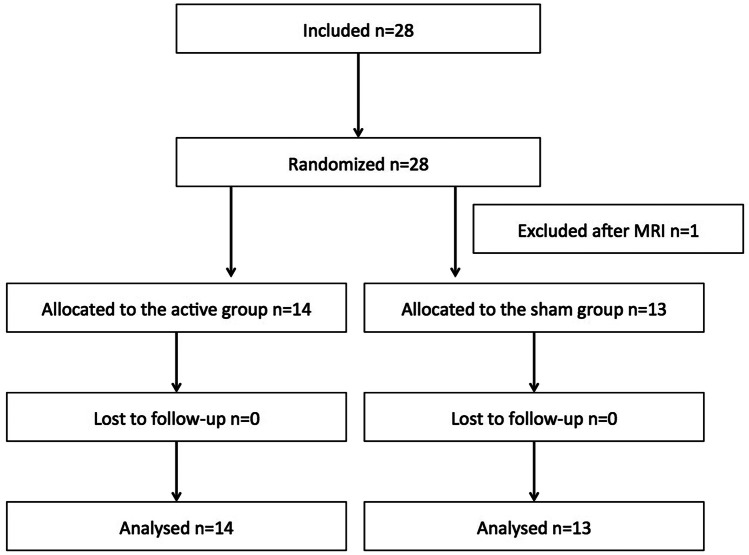
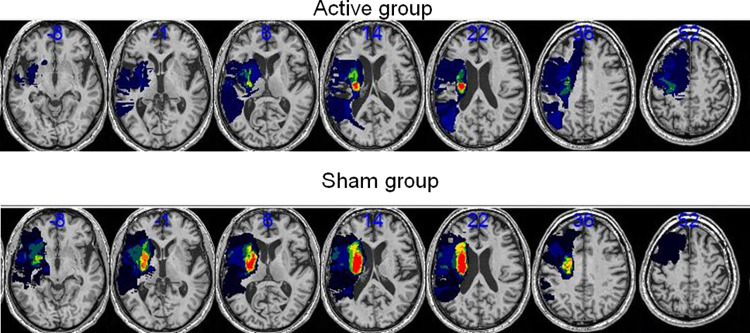
Between October 2015 and October 2018, 28 patients were included and randomized in the study. Twenty-seven patients were analyzed (Fig. 1). There was no drop-out during the study. Figure 2 displays the location of the infarct lesions. The patient characteristics are presented in Table 1. There was no significant difference between the two groups (active or sham) for the three variables minimized in the algorithm (age, UL-Fugl-Meyer, and side of the lesion). There was no TIME*GROUP interaction in the JTT (F(2, 50): 1.2, p: 0.28) nor the GS performances (F(2, 50): 0.7, p: 0.48) of the unaffected hand confirming that the change in JTT/GS for the unaffected hand between the sham and active groups was similar. The subsequent analyses were performed with the ratios of the affected/unaffected hands (JTTr and GSr).
Fig. 1.
Flowchart of the study. Twenty-eight patients were included in the study and randomized and twenty-seven patients were analyzed (one exclusion after MRI since the hand knob area was infarcted)
Fig. 2.
Lesion probability maps for the active (top) and sham (bottom) cohorts overlaid on a normalized T1. The color map reflects the percentage of lesioned voxels. For the two groups, all lesions are fixed to the left hemisphere. Left is left. Numbers indicate the coordinates in the MNI space
Table 1.
Characteristics at inclusion
| Active group N = 14 | Sham group N = 13 | p-value | |
|---|---|---|---|
| Age (years) | 63 + − 14 | 60 + − 11 | 0.48 |
| Gender (male, n%) | 11 (78%) | 10 (77%) | 0.68 |
| Handedness | 100% right handed | 100% right handed | 0.52 |
| Time post stroke (months) | 202 ± 355 | 374 ± 481 | 0.29 |
| Side of the lesion | 8 left (57%) | 7 left (54%) | 0.81 |
| Location | 2 C, 9 SC, 3 CSC | 10 SC, 3 CSC | 0.36 |
| UL-Fugl-Meyer score | 43 ± 14 | 38 ± 19 | 0.39 |
Data are presented as mean and standard deviation
C cortical, SC subcortical, and CSC cortico-subcortical
The other baseline clinical characteristics (Table 1) including the JTTr and GSr (p: 0.37 and 0.20), the electrophysiological data (MEPr p: 0.48) (Table 2), and MRI data (FAratio for the dentato-thalamo-cortical tract p: 0.70; FAratio for the corticospinal tract: p: 0.69; ipsilesional M1 activity p: 0.06) were similar between the two groups. Five individuals were MEP negative. MEP negativity was stable for all course of treatment.
Table 2.
Mean and SD amplitude of MEP ratio before the intervention (B – D0), immediately at the end of the treatment (D5), and a 1 month later (D30) in both the active and the sham groups
| Active group | Sham group | |||||
|---|---|---|---|---|---|---|
| Baseline (D0) | D5 | D30 | Baseline (D0) | D5 | D30 | |
| MEP ratio | 0.44 (0.62) | 0.45 (0.65) | 0.55 (1.09) | 0.27 (0.51) | 0.33 (0.59) | 0.27 (0.44) |
Clinical Efficacy of CER_M1 PAS
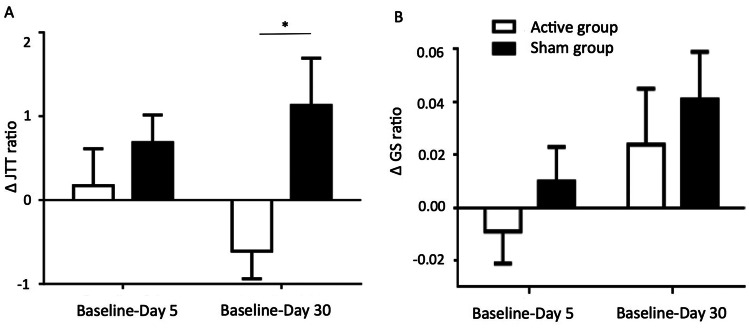
The time course of the JTTr showed a significant GROUP*TIME interaction (Fig. 3, F(1, 26): 3.27, p: 0.04). There was no effect of TIME (F(2, 50): 0.6, p: 0.55) and GROUP (F(1, 25): 1.1, p: 0.29). The change in JTTr score between the active and the sham group was not significant at D5 (p: 0.16), but was at D30 (p: 0.01). In other words, there was a clinical improvement in the JTTr in the active vs. the sham group at D30 (Table 3). The magnitude of change at D30 in the active group was an improvement of 10.3% and in the sham group a worsening of 12.5%.
Fig. 3.
Clinical effects of active vs. sham CER_M1 PAS for the difference in JTT ratio (A) and the difference in GSr (B). Values correspond to mean and standard error of the mean. D5 represents follow-up at day 5 and D30 at one month. *p < 0.05 corresponded to the comparison between ΔJTTr at D30 between the active and the sham groups. Between D0 and D5, JTTr did not improve in both active (p:0.70) and sham (p:0.18) groups. Between D0 and D30, JTTr improved in the active group (mean ΔJTTr: − 0.6 ± 1.2, p: 0.04), while it tended to worsen in the sham group (mean ΔJTTr: 1.1 ± 2.0, p: 0.07)
Table 3.
Mean and SD JTT ratio and GS ratio before the intervention (B – D0), immediately at the end of the treatment (D5), and a 1 month later (D30) in both the active and the sham groups
| JTT ratio | GS ratio | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Active group | ||||
| Baseline (D0) | 5.92 | 6.95 | 0.37 | 0.27 |
| D5 | 6.00 | 7.28 | 0.48 | 0.24 |
| D30 | 5.31 | 6.66 | 0.53 | 0.27 |
| Sham group | ||||
| Baseline (D0) | 9.03 | 11.7 | 0.37 | 0.26 |
| D5 | 9.71 | 10.59 | 0.38 | 0.26 |
| D30 | 10.14 | 12.38 | 0.41 | 0.29 |
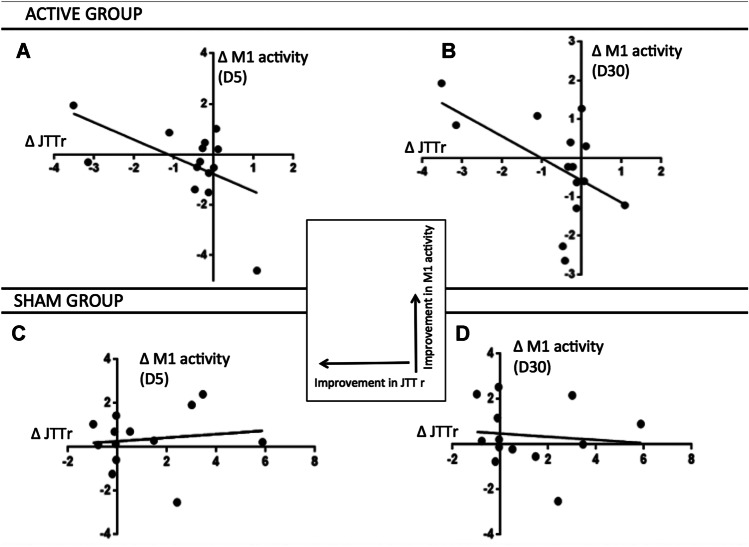
Clinical improvement in the CER_M1 PAS active group (ΔJTTr_D30) was associated with changes in functional ipsilesional M1 activity measured by fMRI (Fig. 4). The larger the clinical improvement at D30, the larger the increase in ipsilesional M1 activity at D30 (correlation coefficient: − 0.535, 95%CI: − 0.829; − 0.007, p: 0.04). The same relationship between changes in M1 activity between D0 and D5, and clinical improvement at D30 was found (correlation coefficient: − 0.554, 95%CI: − 0.838; − 0.033, p: 0.04), suggesting that the changes in M1 activity preceded the clinical improvement. This correlation was not found in the sham group (p: 0.68 at D5, p: 0.74 at D30).
Fig. 4.
Scatter plots of the clinical improvement (x-axis) as assessed by changes in the JTTr between D30 and baseline (D0) and changes in ipsilesional M1 activity (y-axis) between D5 and baseline (A, C) and, as well as between D30 and baseline (B, D). A and B refer to the active group and C and D to the sham group. The equations are: A y = − 0.68x − 0.77, p: 0.04; B y = − 0.56x − 0.57, p: 0.04; C y = − 0.08x + 0.27, p: 0.24; D y = − 0.07x + 0.48, p: 0.30
However, clinical improvement in the CER_M1 PAS active group (ΔJTTr_D30) was not explained by changes in corticospinal excitability as assessed by TMS-induced MEP amplitude ratio (p: 0.49 at D5, p: 0.87 at D30).
Considering the GSr, there was no effect of treatment (GROUP*TIME interaction: F(1.25): 0.60; p: 0.54) (Fig. 3). There were no correlations between ΔGSr and changes in M1 ipsilesional activation or corticospinal excitability at any time point.
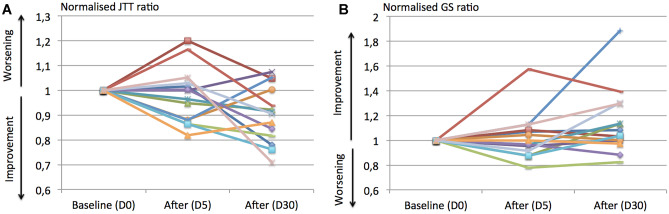
Figure 5 displays the individual scatter plots of JTTr and GSr in the active group.
Fig. 5.
Individual scatter plots of the JTT r and GSr in the active group before (D0) and after (D5 and D30) the stimulation. JTTr (A) and the GSr (B) are normalized to the baseline score
Supplemental Material Table 1 summarizes the values of the neuroimaging data.
Biomarkers of Treatment Response
The factor at D0 that correlated to JTTr improvement at D30 in the active treated group was the integrity of the CST (FAratio; correlation coefficient: − 0.602; 95%CI: − 0.858; − 0.106, p: 0.02). There was only a trend for the integrity of the dentato-thalamo-cortical tract to correlate with JTTr improvement (FAratio: correlation coefficient = − 0.505, 95%CI: − 0.816; 0.034, p: 0.06). In other words, the more preserved the pathways, the more effective the treatment.
For the sham group, there were no correlation between improvements in JTTr and the integrity of the two tracts (p > 0.05).
Safety Outcome
None of the subjects experienced noticeable adverse effects during or after the study except transient cephalalgia (n = 1) in each group and one reflex syncope right after the end of the stimulation at D5 in the sham group. Nevertheless, five patients reported discomfort in the active group following the intervention, while two patients reported discomfort in the sham group. Indeed, the cerebellar coil was placed laterally (3 cm right or left from the inion), and one wing of the coil was closed to the masseter muscle leading to masseter contractions every pulse.
Discussion
This randomized, sham-controlled, double-blind pilot trial suggested that active CER_M1 PAS might be associated with better hand function in stroke patients. This also suggested that stimulation of the cerebellum pathway is relatively specific since improvement was only seen in a score involving coordination and dexterity (JTT), but not the grip strength (GS). Moreover, the task-related increase of ipsilesional M1 activation between before and after the PAS treatment was associated with the extent of the clinical score improvement (JTTr) for patients in the active treated group. The inter-individual variability in clinical improvement was partly explained by the structural integrity of the efferent (corticospinal tract) pathway engaged in the PAS stimulation with a trend for the structural integrity of the (dentato-thalamo-cortical tract) afferent pathway.
How to Explain the Efficacy of the CER_M1 PAS?
The efficacy of CER_M1 PAS was concordant with previous preclinical studies [22]. Machado et al. [23] delivered a 20 Hz electrical stimulation of the contralesional lateral cerebellar nucleus in rats and demonstrated an improvement in the Montoya staircase task that assessed skilled paw reaching. Moreover, in another study, repeated lateral cerebellar nucleus stimulation using optogenetics resulted in a robust and persistent recovery on the rotating beam test. Indeed, the stimulated rats showed increased expression of the GAP42 protein, responsible for axonal growth in the ipsilesional sensorimotor cortex [24]. This suggests that the cerebello-motor loop stimulation drives morphologic changes of the efferent pathway. Our study is also in line with human studies such as the promising results obtained by Koch et al. [25] that showed improved gait and balance recovery following repetitive TMS over the cerebellum in stroke patients. Additionally, the first-in-human trial (EDEN study, NCT #02,835,443), aimed at assessing the safety and feasibility of invasive deep brain stimulation of the dentate nucleus for improving upper limb motor function, started in 2016 [26].
In line with the aforementioned studies, the first explanation of our results is that CER_M1 PAS could strengthen the synaptic transmission between the cerebellum and the primary motor cortex. This view is supported by the fact that the integrity of the CST tract, which provides the anatomical support of this loop, is associated with clinical improvement. Even if how PAS modulates cortical synaptic plasticity is not exactly known, PAS induces both rapid and long-term effects in the primary motor cortex [27]. PAS modulates rapidly the cortical excitability of the primary motor cortex, which could be the explanation why activity in the primary motor cortex increased in treated patients who benefited the most. On the other hand, PAS effects can be blocked by NMDA receptor antagonist, suggesting long-term potentiation like phenomenon at play [28]. In this context, it is possible that delayed effects of CER_M1 PAS are related to synaptic plasticity processes and consolidate over time [29].
The second possibility is that CER_M1 PAS treatment involves proprioceptive and/or somato-sensory afferences for two reasons [30]. First, the cerebellum is indirectly connected to these systems in the brainstem in such a way that the stimulation could indirectly lead to the activation of this system. Second, the double-cone coil, when placed laterally on the cerebellum, has one of its wings on the upper part of the neck. However, this possibility is unlikely given that (i) we kept the conditioned stimulus intensity relatively low (below 50% of the maximum stimulator output) and (ii) the inter-stimulus interval used was very short (2 ms) which is not compatible with the involvement of a proprioceptive or sensitive component.
Finally, the third explanation is that the effect is mediated by the direct stimulation of M1 and not the cerebellum. However, the sham stimulation, which consists of the same M1 stimulation than the active CER_M1 PAS but without the cerebellum activation, was not associated with M1 activity changes. Though, it is unlikely that the changes in M1 relied on the M1 stimulation only but was specific to the cerebello-motor loop activation.
CER_M1 PAS-Induced Changes in the Brain
Patients who benefited the most from the CER_M1 PAS, as assessed by JTTr, were those who exhibited increased activation of the ipsilesional M1 at the end of treatment (D5) and later (D30) with respect to pre-treatment levels. This suggests a better integration and processing of information within this area. In addition, given that this association occurred as soon as D5, the improvement related to M1 reorganization might begin at the end of the intervention (and before the clinical improvement), a hypothesis compatible with the well-documented mechanisms of PAS protocols on synaptic transmission.
At first glance, clinical improvement without change in MEP amplitude may be surprising, but has been previously described in stroke patients [31]. In healthy subjects, anodal tDCS has been found to increase the inhibitory influence exerted by the cerebellum over the primary motor cortex without effect on MEP [32]. In addition, Daskalakis et al. [33] have suggested, in a study investigating the interaction between the cerebello-motor loop and parameters assessing M1 excitability, that the cerebellar output activated by the TMS stimulation terminates mainly on M1 interneurons, which are not immediately contributing to the MEP. This hypothesis is consistent with our fMRI results and the lack of change in MEP amplitude.
Limitations
Our main limitation is the small sample size of the study. Our findings need to be confirmed in future trials, including a larger sample size, to draw up compelling conclusions. Our sample size did not allow reliable subgroups analysis for example between MEP positive (n = 22) vs. negative individuals (n = 5) or between patients enrolled at the subacute (≤ 3 months, n = 11) vs. chronic phase (> 3 months, n = 16).
The TMS intensity we used to stimulate the cerebellum is a second shortcoming. Indeed, it is well-known that cerebellar-brain inhibition intensity depends on the intensity of both conditioning and test pulses [34]. The higher the intensity of the stimuli, the larger the cerebellar-brain inhibition. In stroke patients, the resting motor threshold on the affected hemisphere is higher than that observed on the unaffected one or on the healthy population. But the discomfort induced by higher cerebellar stimulation intensity using a similar double cone-coil prevented from going to 60–70% of the maximal stimulator output [35]. We chose to maintain the TMS intensity applied over the cerebellum to 50% of the maximal stimulator output because higher intensity leads to masseter contractions and clenched teeth that can be painful for patients. In line with this, Kassavetis et al. [36] reported that seven out of 16 participants could not complete their study, as they found cerebellar stimulation to be too uncomfortable. Third, we found a correlation between changes in M1 activity and treatment-related improvement, but this needs to be confirmed in larger sample sizes, as the correlation might be driven by the patients who benefited the most from the intervention. Finally, caution must be taken by interpreting our results given that the magnitude of change of the dexterity task was small (10.3% of improvement in the active group and 12.5% of worsening in the sham group) so that their clinical relevance might be questioned.
In conclusion, CER_M1 PAS combined with physical training might be a promising approach to enhance hand function in stroke patients. Increased ipsilesional M1 activation preceded functional improvements as assessed by the Jebsen-Taylor test in patients who benefited the most from the CER_M1 PAS protocol suggesting that this might be one of the neural substrate mediating the PAS-induced after-effects. The results of this study also confirm the role of the structural integrity of input and output pathways to mediate the CER_M1 PAS effect. However, this is a pilot study looking to explore a novel PAS cerebello-motor protocol, which may help plan a larger clinical efficacy study.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Clinical Investigation Center for Neurosciences (CIC-1422, Inserm, Pitié-Salpêtrière Hospital, Paris) and M. Maier for his assistance with the fMRI paradigm and material. We thank Eric Moulton for his English proofreading.
Funding
The research leading to these results has received funding from “Investissements d’avenir” ANR-10-IAIHU-06.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kandel M, Beis JM, Le Chapelain L, Guesdon H, Paysant J. Non-invasive cerebral stimulation for the upper limb rehabilitation after stroke: a review. Ann Phys Rehabil Med. 2012;55:657–680. doi: 10.1016/j.rehab.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Hsu WY, Cheng CH, Liao KK, Lee IH, Lin YY. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke: a meta-analysis. Stroke. 2012;43:1849–1857. doi: 10.1161/STROKEAHA.111.649756. [DOI] [PubMed] [Google Scholar]
- 3.Hao Z, Wang D, Zeng Y, Liu M. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database of Systematic Reviews 2013(5);CD008862. 10.1002/14651858.CD008862.pub2 [DOI] [PMC free article] [PubMed]
- 4.Wessel MJ, Hummel FC. Non-invasive cerebellar stimulation : a promising approach for stroke recovery ? Cerebellum. 2018;17:359–371. doi: 10.1007/s12311-017-0906-1. [DOI] [PubMed] [Google Scholar]
- 5.Nordmann G, Arizona V, Langguth B, Schecklmann M. A systematic review of non-motot rTMS induced motor cortex plasticity. Front Hum Neurosci. 2015;21:416. doi: 10.3389/fnhum.2015.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sokolov AA, Miall RC, Ivry RB. The cerebellum: adaptive prediction for movement and cognition. Trends Cogn Sci. 2017;21:313–332. doi: 10.1016/j.tics.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mintzopoulos D, Astrakas LG, Khanicheh A, et al. Connectivity alterations assessed by combining fMRI and MR-compatible hand robots in chronic stroke. Neuroimage. 2009;47(Supp 2):T90–97. doi: 10.1016/j.neuroimage.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lu J, Liu H, Zhang M, et al. Focal pontine lesions provide evidence that intrinsic functional connectivity reflects polysynaptic anatomical pathways. J Neurosci. 2011;31:15065–15071. doi: 10.1523/JNEUROSCI.2364-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Yu C, Chen H, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- 10.Rosso C, Valabregue R, Attal Y, et al. Contribution of corticospinal tract and functional connectivity in hand motor impairment after stroke. PLoS ONE. 2013;27(8):e73164. doi: 10.1371/journal.pone.0073164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulz R, Frey BM, Koch P, et al. Cortico-cerebellar structural connectivity is related to residual motor output in chronic stroke. Cereb Cortex. 2017;27(1):635–645. doi: 10.1093/cercor/bhv251. [DOI] [PubMed] [Google Scholar]
- 12.Müller Dahlhaus F, Ziemann U, Classen J. Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front Hum Neurosci. 2010;2:34. doi: 10.3389/fnsyn.2010.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carson RG, Kennedy NC. Modulation of human corticospinal excitability by paired associative stimulation. Front Hum Neurosci. 2013;7:823. doi: 10.3389/fnhum.2013.00823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu MK, Tsai CH, Ziemann U. Cerebellum to motor cortex paired associative stimulation induced bidirectional STDP-like plasticity in human motor cortex. Front Hum Neurosci. 2012;6:260. doi: 10.3389/fnhum.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ugawa Y, Uesaka Y, Terao Y, Hanajima R, Kanazawa I. Magnetic stimulation over the cerebellum in humans. Ann Neurol. 1995;37:703–713. doi: 10.1002/ana.410370603. [DOI] [PubMed] [Google Scholar]
- 16.Yelnik AP, Quintaine V, Andriantsifanetra C, et al. AMOBES (active mobility very early after stroke) Stroke. 2017;48:400–405. doi: 10.1161/STROKEAHA.116.014803. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. The safety of TMS Consensus Group. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–2039. [DOI] [PMC free article] [PubMed]
- 18.Ugawa Y, Day BL, Rothwell JC, Thompson PD, Merton PA, Marsden CD. Modulation of motor cortical excitability by electrical stimulation over the cerebellum in man. J Physiol. 1991;441:57–72. doi: 10.1113/jphysiol.1991.sp018738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hummel F, Cohen LG. Improvement of motor function with noninvasive cortical stimulation in a patient with chronic stroke. Neurorehabil Neural Repair. 2005;19:14–19. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- 20.Kemlin C, Moulton E, Lamy JC, et al. Elucidating the structural and functional correlates of upper-limb post-stroke motor impairment. Stroke. 2019;50:3647–3649. doi: 10.1161/STROKEAHA.119.027126. [DOI] [PubMed] [Google Scholar]
- 21.Moulton E, Galléa C, Kemlin C, et al. Cerebello-cortical differences in effective connectivity of the dominant and non-dominant hand during a visuomotor paradigm of grip force control. Front Hum Neurosci. 2017;11:511. doi: 10.3389/fnhum.2017.00511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miterko LN, Baker KB, Beckinghausen J, et al. Consensus paper: experimental neurostimulation of the cerebellum. Cerebellum. 2019;18:1064–1097. doi: 10.1007/s12311-019-01041-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machado AG, Baker KB, Schuster D, Butler RS, Rezai A. Chronic electrical stimulation of the contralesional lateral cerebellar nucleus enhances recovery of motor function after cerebral ischemia in rats. Brain Res. 2009;1280:107–116. doi: 10.1016/j.brainres.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah AM, Ishizaka S, Cheng MY, et al. Optogenetic neuronal stimulation of the lateral cerebellar nucleus promotes persistent functional recovery after stroke. Sci Rep. 2017;7:46612. doi: 10.1038/srep46612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch G, Bonni S, Casula EP, Iosa M, Paolucci S, Pellicciari MC. Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke. JAMA Neurol. 2019;76:170–178. doi: 10.1001/jamaneurol.2018.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wathen CA, Frizon LA, Maiti TK, Baker KB, Machado AG. Deep brain stimulation of the cerebellum for post-stroke motor rehabilitation. Neurosurg focus. 2018;45:E13. doi: 10.3171/2018.5.FOCUS18164. [DOI] [PubMed] [Google Scholar]
- 27.Lefaucheur JP. Neurophysiology of cortical stimulation. Int Rev Neurobiol. 2012;107:57. doi: 10.1016/B978-0-12-404706-8.00005-X. [DOI] [PubMed] [Google Scholar]
- 28.Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krakauer JW, Shadmehr R. Consolidation of motor memory. Trends Neurosci. 2006;29:58–64. doi: 10.1016/j.tins.2005.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popa T, Russo M, Meunier S. Long-lasting inhibition of cerebellar output. Brain Stimul. 2010;3:161–169. doi: 10.1016/j.brs.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Hummel F, Celnik P, Giraux P, et al. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- 32.Galea JM, Jayaram G, Ajagbe L, Celnik P. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–9122. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Daskalakis ZJ, Paradiso GO, Christensen BK, Fitzgerald PB, Gunraj C, Chen R. Exploring the connectivity between the cerebellum and motor cortex in humans. J Physiol. 2004;557:689–700. doi: 10.1113/jphysiol.2003.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez L, Major BP, Teo W-P, Byrne LK, Enticott PG. The impact of stimulation intensity and coil type on reliability and tolerability of cerebellar brain inhibition (CBI) via dual-coil TMS. Cerebellum. 2018;17:540–549. doi: 10.1007/s12311-018-0942-5. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez L, Major BP, Teo WP, Byrne LK, Enticott PG. Assessing cerebellar brain inhibition (CBI) via transcranial magnetic stimulation (TMS): a systematic review. Neurosci Biobehav Rev. 2018;86:176–206. doi: 10.1016/j.neubiorev.2017.11.018. [DOI] [PubMed] [Google Scholar]
- 36.Kassavetis P, Hoffland BS, Saifee TA, Bhatia KP, Van De Warrenburg BP, Rothwell JC. Cerebellar brain inhibition is decreased in active and surround muscles at the onset of voluntary movement. Exp Brain Res. 2011;209:437–442. doi: 10.1007/s00221-011-2575-5. [DOI] [PubMed] [Google Scholar]
- 37.Groppa S, Oliviero A, Eisen A, et al. A practical guide to diagnostic TMS: report of an IFCN committee. Clin Neurophysiol. 2012;123:858–882. doi: 10.1016/j.clinph.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simis M, Di, Lazzaro V, Kirton A, et al Neurophysiological measurements of affected and unaffected motor cortex from a cross-sectional, multi-center individual stroke patient data analysis study. Neurophysiol Clin. 2016;46:53–61. doi: 10.1016/j.neucli.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Lindberg PG, Roche N, Robertson J, Roby-Brami A, Bussel B, Maier MA. Affected and unaffected quantitative aspects of grip force control in hemiparetic patients after stroke. Brain Res. 2012;1452:96–107. doi: 10.1016/j.brainres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage. 2003;20:870–888. doi: 10.1016/S1053-8119(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 41.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Moulton E, Valabrègue R, Díaz B, et al. Comparison of spatial normalization strategies of diffusion MRI data for studying motor outcome in subacute-chronic and acute stroke. Neuroimage. 2018;183:186–199. doi: 10.1016/j.neuroimage.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 43.Newton JM, Ward NS, Parker GJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas - Relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.