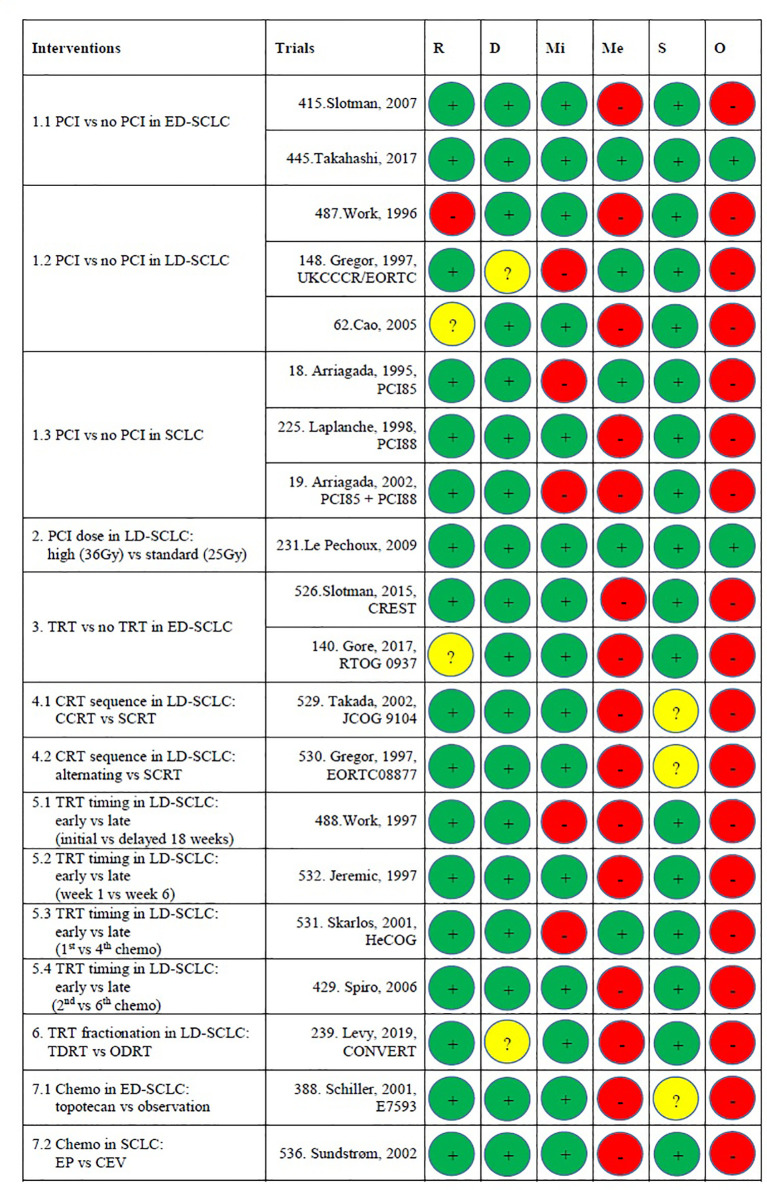
Figure 2.
Risk of bias assessments. Risk of bias legend. R, Bias arising from the randomization process; D, Bias due to deviations from intended interventions; Mi, Bias due to missing outcome data; Me, Bias in measurement of the outcome; S, Bias in selection of the reported results; O, Overall risk of bias. Domain 1: Risk of bias arising from the randomization process: The study conducted by Work et al. (34) was at high risk of bias because PCI vs no PCI was not strictly randomized. The study conducted by Cao et al. had “some concerns” because of no information about the random allocation sequence. RTOG 0937 had “some concerns” because baseline age was unbalanced between arms (P = 0.03). The other 16 studies were assessed as at low risk of bias. Domain 2: Risk of bias due to deviations from the intended interventions (effect of assignment to intervention): The CONVERT trial was assessed to have “some concerns” because it is unclear whether there were deviations from the intended intervention that arose because of the trial context. The UKCCCR/EORTC trial was assessed to have “some concerns” since there were deviations from the intended intervention that arose because of the trial context. The others were at low risk. Domain 3: Missing outcome data: This domain is difficult to tell because most trials did not have a regular brain CT/MRI scan plan during the follow-up. In the trials that did have a pre-planned brain CT/MRI scan schedule, only one trial (IPC85) mentioned the compliance at some time point. Readers do not know how many data were missing. The UKCCCR/EORTC trial and HeCOG were at high risk because of no information about missing data. IPC85, the pooled analysis of IPC85+ IPC88, and the study conducted by Work et al. (35) were at high risk because many data were missing but there were no evidence that the result was not biased by missing data. The other 14 studies were at low risk. Domain 4: Risk of bias in measurement of the outcome: 14 studies were judged to be at high risk because the method of measuring the outcome (BM) was inappropriate. They performed brain MRI/CT when patients experience neurological symptoms. The other five trials were at low risk because they had pre-planned brain MRI/CT scan during follow-up. Domain 5: Risk of bias in selection of the reported result: JCOG 9104, E7593, and the trial conducted by Gregor et al. (EORTC) had “some concerns” because of no information about pre-specified analysis plan or selection from multiple eligible analyses. Overall risk of bias: Only the studies conducted by Le Pechoux et al. and Takahashi et al. were judged to be at low risk of bias. The other 17 trials were judged as high risk of bias. This is mainly because of domains 3 and 4. CCRT, concurrent chemoradiotherapy; CEV, cyclophosphamide–epirubicin–vincristine; chemo, chemotherapy; CRT, chemoradiotherapy; ED, extensive-stage disease; EP, etoposide-platinum; LD, limited-stage disease; ODRT, once-daily radiotherapy; PCI, prophylactic cranial irradiation; SCLC, small cell lung cancer; SCRT, sequential chemoradiotherapy; TDRT, twice-daily radiotherapy; TRT, thoracic radiotherapy.