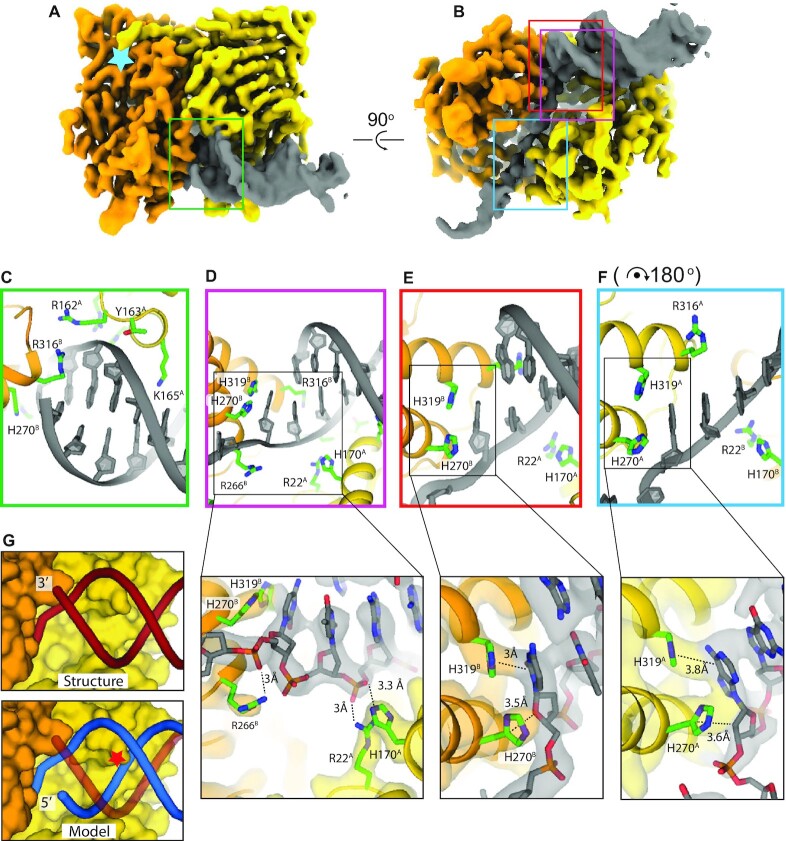
Figure 2.
Structure of MutL bound to a 3′ resected DNA end. (A) front view and (B) bottom view of the cryo-EM map of the MutL ATPase domain dimer bound to a 3′ resected primer-template junction. Monomer A is coloured in yellow, monomer B in orange. Light blue star represents the position of the ATP binding pocket. Coloured squares mark the areas of the close ups shown in (C)–(F). (C) Backbone binding of the double stranded section. (D) Binding of the ds/ssDNA junction with additional close up of the interaction between R22, H170 and R266 with the phosphates of the DNA. (E) ds/ssDNA binding with additional close up on the interaction between H270 and H319 and the first unpaired nucleotide of the continuous strand. (F) Binding to the single stranded overhang that shows a similar interaction between histidines H270 and H319 and an unpaired base. For easy comparison, the panel is rotated 180° with respect to panel (E). Close ups in figure (D), (E) and (F) display the experimental cryo-EM map coloured relatively to the chains. (G) Comparison between the structure (top) and a predicted model of a 5′ resected DNA substrate after superposition of the continuous strand (bottom). Red star indicates the clash of the backbone of the 5′ resected strand with K165 and the helix it is located on.