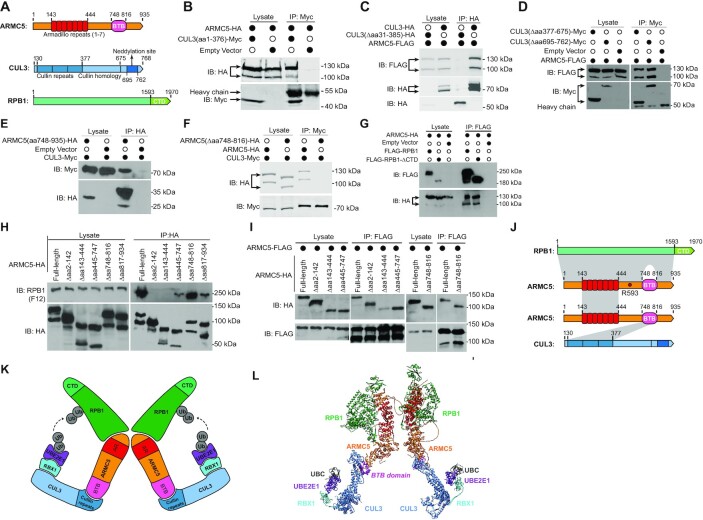
Figure 2.
Identification of the regions of interaction in ARMC5, CUL3, and RPB1 molecules. (A) Schematics of key domains of human ARMC5, CUL3 and RPB1 molecules. (B) The cullin repeats of CUL3 interacted with ARMC5. HEK293 cells were transfected with plasmids expressing CUL3 N-terminal cullin repeats (CUL3(aa1–376)-Myc) and full-length ARMC5-HA. The lysates were precipitated with anti-Myc Ab and blotted with anti-HA and anti-Myc Abs. (C) CUL3 with cullin repeats deleted no longer interacted with ARMC5. HEK293 cells were transfected plasmids expressing either full-length CUL3 or CUL3 with culling repeats deleted (CUL3(Δaa31–385)-HA) and full-length ARMC5-HA. The lysates were precipitated with anti-HA Ab and blotted with anti-FLAG and anti-HA Abs. (D) The CUL3 cullin homology domain and its C-terminal fragment were not necessary for interaction with ARMC5. HEK293 cells were transfected plasmids expressing CUL3 with the cullin homology domain deleted (CUL3(Δaa377–676)-Myc) or with its C-terminal sequence, including the neddylation site deleted (CUL3(Δaa 695–762)-Myc), and full-length ARMC5-FLAG. The lysates were precipitated with anti-Myc Ab and blotted with anti-FLAG and anti-Myc Abs. (E) The ARMC5 C-terminal sequence containing the BTB domain was sufficient to interact with CUL3. HEK293 cells were transfected plasmids expressing CUL3-Myc and the ARMC5 C-terminal sequence containing the BTB domain plus the following 119-aa sequence (ARMC5(aa748–935)-HA). The lysates were precipitated with anti-HA Ab and blotted with anti-HA or anti-Myc Abs. (F) ARMC5 without BTB domain no longer bound to CUL3. HEK293 cells were transfected with plasmids expressing CUL3-Myc and BTB domain-deleted ARMC5-HA (ARMC5(Δaa748–816)-HA). The lysates were precipitated with anti-Myc Ab and blotted with anti-HA or anti-Myc Abs. (G) The CTD of RPB1 was not essential for the association between RPB1 and ARMC5. HEK293 cells were transfected plasmids expressing ARMC5-HA and full-length RPB1 (FLAG-RPB1) or RPB1 with its CTD deleted (FLAG-RPB1-ΔCTD). The lysates were precipitated with anti-FLAG Ab and blotted with anti-HA or anti-FLAG Abs. (H) The N-terminal sequence (aa2–142) before the ARM domain (aa143–444) and the ARM domain of ARMC5 were both needed for RPB1 binding. The sequence after the ARM domain and before the BTB domain, and the sequence after the BTB domain also contributed to RPB1 binding but to a lesser degree. HEK293 cells were transfected plasmids expressing HA-tagged ARMC5 deletion mutants as described. The lysates were precipitated with anti-HA Ab and blotted with anti-RPB1 (clone F12) or anti-HA Abs. (I) ARMC5 interacted with ARMC5 through their ARM domains (positions aa 143-444). FLAG-tagged full-length ARMC5 was transfected into HEK293 cells along with HA-tagged full-length ARMC5 or deletion mutants. The lysates were immunoprecipitated with anti-FLAG Ab and blotted with anti-HA or anti-FLAG Abs. (J) A schematic showing the regions that contributed to the interaction among RPB1, ARMC5, and CUL3. The grey shading between the molecules represents the interaction regions. The lighter shade between RPB1 and ARMC5 indicates a lesser contribution of the regions to the association between these two molecules. The position of ARMC5 R593, which is mutated in Adelaide PBMAH patients, is indicated. (K) A 2D schematic of the novel dimeric RPB1-specific E3. (L) A 3D model of the novel dimeric RPB1-specific E3. In all the experiments, empty vectors were used in transfection as controls. The lysates were also immunoblotted to confirm that the transfected proteins were present. All the experiments were conducted more than three times, and representative results are shown.