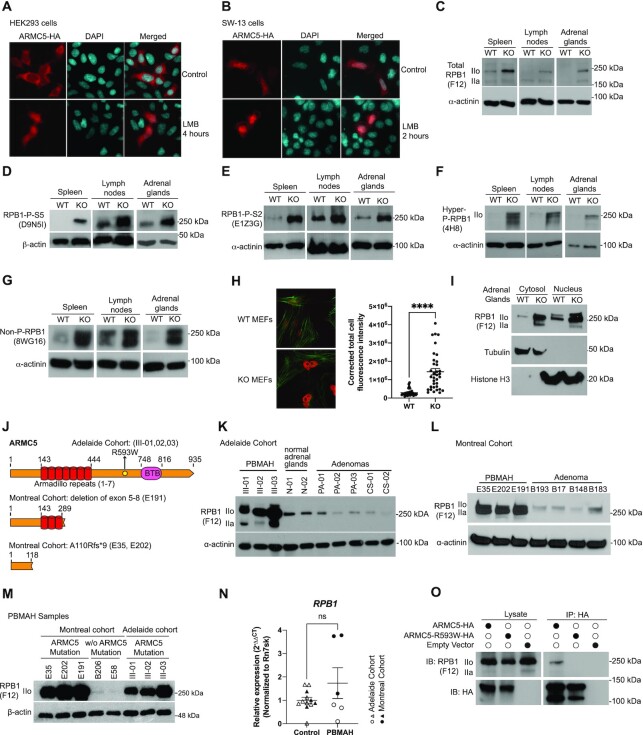
Figure 3.
ARMC5 KO or mutation led to RPB1 accumulation. (A and B) ARMC5 was presented in both the cytosol and nuclei of HEK293 cells (A) and human adrenal carcinoma SW-13 cells (B). Both types of cells were transfected with plasmids expressing ARMC5-HA. The cells were harvested after 36 h and were stained with anti-HA Ab (pseudo-red) and DAPI (pseudo-cyan). In some cultures, nuclear export inhibitor leptomycin B (LMB; 20 nM) was present for the last 2 or 4 h of culture, as indicated. (C–G) Accumulation of RPB1 in KO tissues. The spleen, lymph node, and adrenal gland protein of KO and WT mice were assessed by immunoblotting for total RPB1 (C); mAb clone F12 against the N-terminal sequence of RPB1), RPB1 with phosphorylated S5 in CTD (D); mAb clone D9N5I), RPB1 with phosphorylated S2 in CTD (E); mAb clone E1Z3G), hyper- and hypo-phosphorylated RPB1 (F); mAb clone 4H8), and non-phosphorylated RPB1 (G): mAb clone 8WG16). β-actin or α-actinin was blotted as a loading control. (H) Elevated RPB1 protein (red) expression in the nuclei of KO MEFs, according to immunofluorescence using rabbit anti-RPB1 Ab (D8L4Y) followed by Alexa Fluor™ 555-conjugated goat anti-rabbit IgG. Filamentous actin (green) was stained with Alexa Fluor™ 488 Phalloidin. Representative micrographs are shown on the left. A bar graph on the right shows the means ± SD of corrected total cell fluorescent intensity (CTCF), which is derived from RPB1 signals of 35 WT and 35 KO MEFs from three independent experiments. ****P < 0.0001 (unpaired two-way Student's t-test). (I). Augmented total RPB1 levels (as determined by F12 mAb) in both the cytosolic and nuclei fractions of KO adrenal glands. Cytosolic tubulin and nuclear histone H3 were used as fraction purity and loading controls. (J) Schematics of ARMC5 mutations in the Adelaide and Montreal cohorts. Patient ID numbers are indicated in the parentheses. (K and L) Elevated RPB1 protein expression in the adrenal macronodules from Adelaide (K) and Montreal (L) PBMAH cohorts with germline ARMC5 mutations. Adrenal adenomas or normal adrenals were employed as controls as indicated. Immunoblotting was performed using mAb (clone F12) against total RPB1 protein. (M) Normal RPB1 protein levels in PBMAH adrenals without ARMC5 mutations. The RPB1 protein levels of adrenal macronodules from two PBMAH patients without ARMC5 mutation (Montreal cohort) were compared to that of six adrenal macronodules from PBMAH patients with germline ARMC5 mutations (Montreal and Adelaide cohorts). (N) RPB1 mRNA levels of the Adelaide and Montreal PBMAH adrenal samples with ARMC5 mutations were similar to those of the controls (adrenal adenomas and normal adrenals). (O) The ARMC5 R539W mutation found in the Adelaide cohort resulted in reduced RPB1 association. HEK293 cells were transfected with plasmids expressing WT ARMC5-HA or ARMC5-R539W-HA. Their association with endogenous RPB1 was detected by immunoblotting after immunoprecipitation.