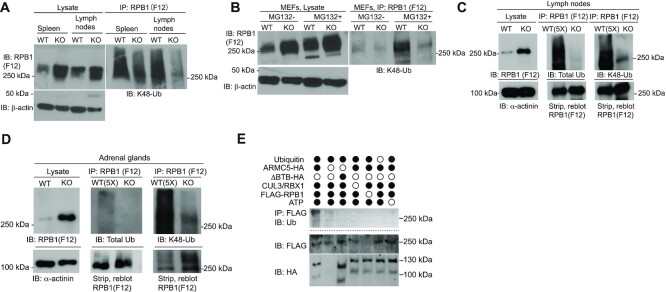
Figure 4.
ARMC5-CUL3-RBX1 as an RPB1-specific E3 based on in vivo and in vitro ubiquitination. (A and B) Reduced K48-linked RPB1 in the KO spleen and lymph nodes (A) and MEFs (B). MEFs were cultured in the absence or presence of proteasome inhibitor MG132 (10 μM) for the last 4 h of culture. β-Actin was blotted for lysate loading control. (C and D) Reduced total and K48-linked RPB1 ubiquitination in KO lymph nodes (C) and adrenal glands (D). Tissue proteins were precipitated with anti-total RPB1 mAb (F12) and immunoblotted with Abs against K48-linked ubiquitin or total ubiquitin, as indicated. α-actinin was blotted for lysate loading control. In (C and D), 5-fold (5×) more WT lysates than the KO counterpart were used as input for immunoprecipitation to detect the weak WT RPB1 ubiquitination signals, using a limited amount of anti-RPB1 Ab during the immunoprecipitation. A similar amount of RPB1 protein in the WT and KO precipitates was shown by immunoblotting. (E) ARMC5-CUL3-RBX1 was a novel RPB1-specific multiple subunit RING-finger E3 according to in vitro ubiquitination assays. Different recombinant proteins, as indicated, were added to the in vitro ubiquitination assay in the presence of E1, E2 (UBE2E1) and ATP. ARMC5 with BTB domain deletion was used as an additional control. The reaction product was immunoprecipitated with anti-FLAG Ab followed by magnetic protein G beads. The immunoprecipitates were blotted with anti-Ub Ab to detect RPB1 ubiquitination. The flow-through of the immunoprecipitation was blotted to confirm the presence of RPB1-FLAG and ARMC5-HA using Abs against these molecules. All the experiments were conducted three times or more, and representative results are shown.