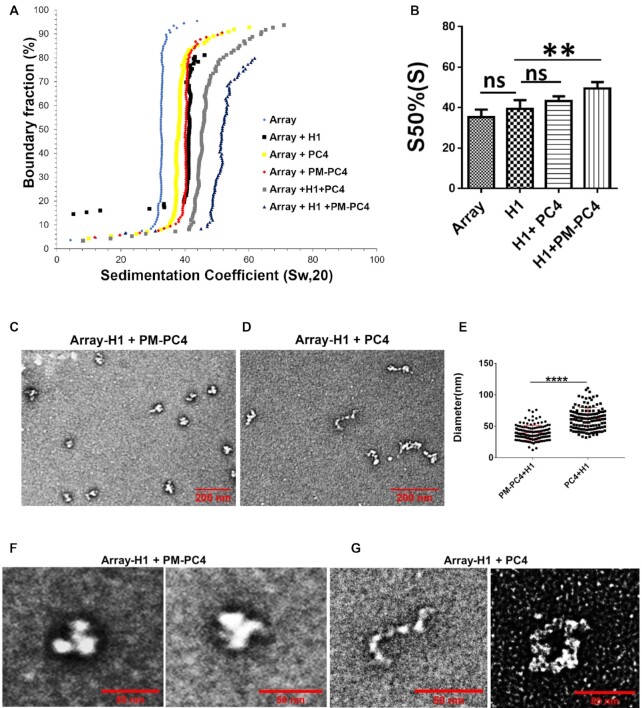
Figure 4.
Phosphorylation enhances PC4 mediated condensation of H1 bound array forming higher order structures. (A) Sedimentation velocity analysis by analytical ultracentrifugation of H1 bound to nucleosome array at 0.5 ratio (H1:NCP) with 1 uM of PM-PC4 (phosphomimic PC4) and PC4 (wild type unmodified recombinant). A nucleosome array without any any ectopic addition of protein was used as a control. (B) Sedimentation coefficient values from the panel A,at a boundary fraction of 50% (n = 3). Data represent the means ± SD.The S50%(S) were statistically analysed by ordinary one-way ANOVA, Sidak's multiple comparisons test as well as Student's paired t-test (*P< 0.05,**P< 0.01,***P< 0.001, ns, non-significant (C, D) EM images showing the overview of the structures formed upon PM-PC4 (phosphomimic PC4) (1 uM) and PC4 (wild type unmodified recombinant) (1 uM) mediated compaction of H1-array respectively. (E) Distribution of diameters (in um) of PM-PC4 (phosphomimic PC4) and PC4 (wild type unmodified recombinant) compacted H1 bound array particles. No. of particles measured = 132. Data represent the means ± SD. The S50%(S) were statistically analysed by Student's unpaired t-test (*P< 0.05,**P< 0.01,***P< 0.001,ns-non-significant). (F, G) The close up view showing the EM image of two H1 bound array compacted by 1uM of PM-PC4 and PC4 respectively.