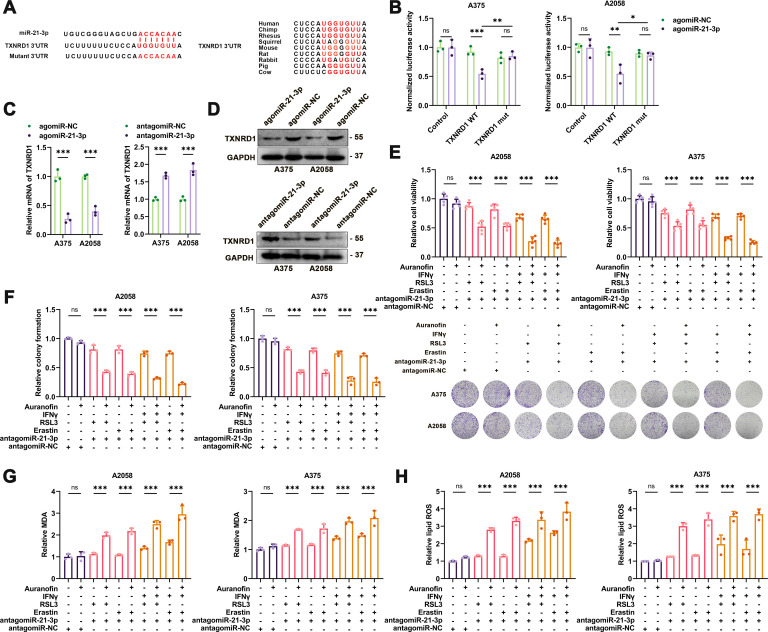
Figure 3.
MiR-21–3p directly targets TXNRD1 to promote ferroptosis. (A) Schematic illustration of the sequence of hsa-miR-21–3p and its complementary sequence in 3’UTR of TXNRD1 mRNA in distinct species. (B) Luciferase activity assays using luciferase reporters with wild-type TXNRD1 3’UTR or mutant TXNRD1 3’UTR were performed with co-transfection of agomiR-21–3p or negative control into A375 and A2058 melanoma cells. (C) Relative mRNA level of TXNRD1 in melanomas with the intervention of miR-21–3p. (D) Immunoblotting analysis of TXNRD1 in melanomas with the intervention of miR-21–3p. (E-F) Relative cell viability and colony formation of ferroptosis inducer-treated melanoma cells with both the intervention of miR-21–3p and pharmacological inhibition of TXNRD1 by Auranofin. (G-H) Relative intracellular MDA content and lipid ROS level in ferroptosis inducer-treated melanoma cells with both the intervention of miR-21–3p and pharmacological inhibition of TXNRD1 by Auranofin. AgomiR-NC, agomiR-21–3p and antagomiR-21–3p were all used at 100 nM in both cell lines. Erastin was used at 10 µM in both cell lines. RSL3 was used at 0.5 µM in A375 and 1 µM in A2058 cell line. Fer-1 was used at 2 µM in both cell lines. IFN-γ was used at 50 ng/mL in both cell lines. Auranofin was used at 2 µM in both cell lines. Data represent the mean±SD of triplicates. P value was calculated by two-tailed Student’s t-test. *P<0.05, **p<0.01, ***p<0.001. IFN, interferon; ns, non-significant; ROS, receiver operating characteristic; TXNRD1, thioredoxin reductase 1; 3’UTR, 3’ untranslated region.