Abstract
A multivalent, bifunctional flagellum carrying two different adhesive peptides in separate flagellin subunits within a filament was constructed in Escherichia coli. The inserted peptides were the fibronectin-binding 115-mer D repeat region of Staphylococcus aureus and the 302-mer collagen-binding region of YadA of Yersinia enterocolitica. Western blotting, immunoelectron microscopy, and adhesion tests with hybrid flagella from an in trans-complemented ΔfliC E. coli strain showed that individual filaments consisted of both recombinant flagellins.
Various bacterial surface display techniques have been applied in basic research to define functional domains in proteins, as well as in vaccinology and biotechnology to present clinically or functionally important peptides (recently reviewed in references 8 and 20). Bacterial surface display techniques are based on genetic in-frame fusion to a gene encoding a carrier protein whose synthesis and translocation onto the cell surface tolerate insertion of a foreign peptide. Outer membrane proteins, lipoproteins, subunits of fimbriae or flagella, and secreted enzymes are examples of carrier proteins successfully applied in gram-negative bacteria; proteins of gram-positive bacteria with the cell wall anchoring motif have been developed as carriers of foreign inserts (8, 20). The bacterial surface display methods so far developed are bifunctional but monovalent in the sense that the fusion partners are expressed in one copy per hybrid molecule. Such hybrids can be composed of two separate toxin subunits (12), two enzymes (3), a viral epitope and bacterial toxin (1), or fusion of a targeting peptide and a toxin (7, 13). Many of the applied bacterial carrier proteins are expressed in multiple copies on the cell surface and could be designed to simultaneously display several foreign peptides. Carrier proteins with multiple biologically active inserts would be advantageous in, e.g., construction of multivalent vaccine strains. The technique described in this report has application in basic research as well as in biotechnology, e.g., in the generation of multivalent vaccines presenting two different epitopes fused to a carrier molecule, construction of targeted effector molecules carrying targeting peptides and effector peptides, and in histological localization of specific tissue domains for diagnostic purposes.
We have expressed adhesive peptides as fusions to the flagellin (FliC) of Escherichia coli (25). By presenting foreign epitopes in thousands of intimately associated copies along the flagellum, a multivalent, high-affinity expression system can be created for a range of applications. FliC, the major constituent of the E. coli flagellar filament, is expressed in 20,000 copies per flagellar filament. The flagellar hook connects the filament to the flagellar basal body and is a polymer of FlgE proteins (for a recent review of flagellar assembly and structure, see reference 5). The N and C termini of FliC form domains involved in subunit-subunit interactions that are important for polymerization and stabilization of the flagellum (16). The central, highly variable region of FliC forms a surface-exposed domain that is responsible for the antigenic variability in flagella (10, 16) and that tolerates large deletions and insertions without loss of flagellar polymerization (9, 11). FliC-based display has been used to express short, 15- to 36-mer, antigenic epitopes for vaccination purposes (reviewed in reference 24). A more recent application is the construction of a library of constrained random dodecapeptides in FliC for the mapping of epitopes for monoclonal antibodies (14). We have shown that hybrid flagella can be successfully applied in the analysis of adhesive domains within bacterial proteins, in localization of their receptor-active domains in tissue sections and cultured mammalian cells, and in raising antiadhesive antibodies (25).
We introduce here bihybrid flagella where two foreign peptides are expressed within the same flagellar filament. We constructed the bifunctional flagella using as model peptides the fibronectin-binding repeats of the FnBPA protein (6, 19) and the collagen-binding fragment of the YadA adhesin (25). The inserts are 115 (D repeats) and 302 (YadA) amino acid residues in size and are expressed as FliC fusions in a conformation exhibiting the adhesive function (25). FnBPA and YadA are important virulence factors that promote bacterial adhesion and invasiveness and hence putative molecular targets for antiadhesive therapy.
We constructed pMB1-based and p15A-based plasmids encoding YadA84-385/FliCΔ and D1,D2,D3/FliCΔ, thus facilitating simultaneous in trans complementation with the two different hybrid genes in the host strain E. coli C600 hsm hsr fliC::Tn10 fimA::cat, also called JT1 (25). The pBluescript-based plasmid pYadA84-385/FliCΔ that encodes collagen-binding hybrid flagellin was available from previous work (25). Compatible, coselectable plasmid pD1,D2,D3/FliCΔ-Km was constructed of pACYC184 (18) by subcloning into the tet gene the 1.75-kb fragment of plasmid pD1,D2,D3/FliCΔ (25) containing the fliC gene fused in frame to the DNA fragment that encodes the D1, D2, and D3 repeats of FnBPA, and by subcloning into the Klenow-treated NcoI site within the cat gene the 2.2-kb Klenow-treated BamHI fragment containing the kanamycin resistance gene of plasmid pHP45Ω-Km (4). The bihybrid complementation strain E. coli(pD1,D2,D3/FliCΔ-Km)(pYadA84-385/FliCΔ) was named BFS1. E. coli JT1 (pFliCΔ) that expresses the deletion derivative of FliCH7 lacking 58 amino acids of the variable region was available from previous work (25) and was used as a control. For production of flagella, strains were grown on Luria plates for 72 h at 28°C with appropriate antibiotics.
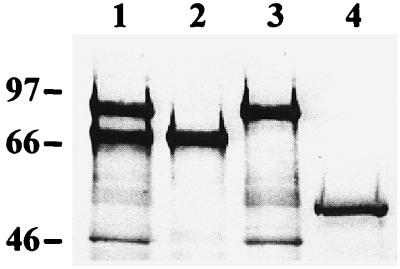
Hybrid flagella were purified, and the FliC content in each flagellar preparation was estimated using image analysis as described before (25). Hybrid flagella were analyzed by electron microscopy after negative staining and by Western blotting using polyclonal anti-H7 flagellum antibodies (25), phosphatase-conjugated secondary antibodies (Dako A/S, Glostrup, Denmark), and a phosphatase substrate solution, essentially as detailed earlier (24). Complementation of the silenced fliC gene in E. coli strain JT1 with each plasmid individually or simultaneously resulted in expression of flagella with normal morphology as assessed by electron microscopy (data not shown). In Western blotting (Fig. 1), the apparent sizes of 69 kDa for D1,D2,D3/FliCΔ and 87 kDa for YadA84-385/FliCΔ corresponded well to their calculated sizes of 67 kDa and 86 kDa, respectively (19, 21). Two equally well expressed, major polypeptides, corresponding in size to D1,D2,D3/FliCΔ and YadA84-385/FliCΔ, were detected in the Western blot of flagella purified from the strain BFS1. The polypeptides of smaller apparent size present in the preparations were apparently flagellar minor proteins and hook proteins also present in flagellar preparations used for immunization. The results showed that both foreign inserts in FliC were expressed simultaneously in BFS1 and polymerized with similar efficiency.
FIG. 1.
Western blot of flagellar constructs with anti-H7 flagellum antibodies. The flagella were bihybrid flagella from E. coli BFS1 (lane 1); D1,D2,D3/FliCΔ flagella (lane 2); YadA84-385/FliCΔ flagella (lane 3); and FliCΔ flagella (lane 4). The positions of molecular mass markers (in kilodaltons) are indicated on the left.
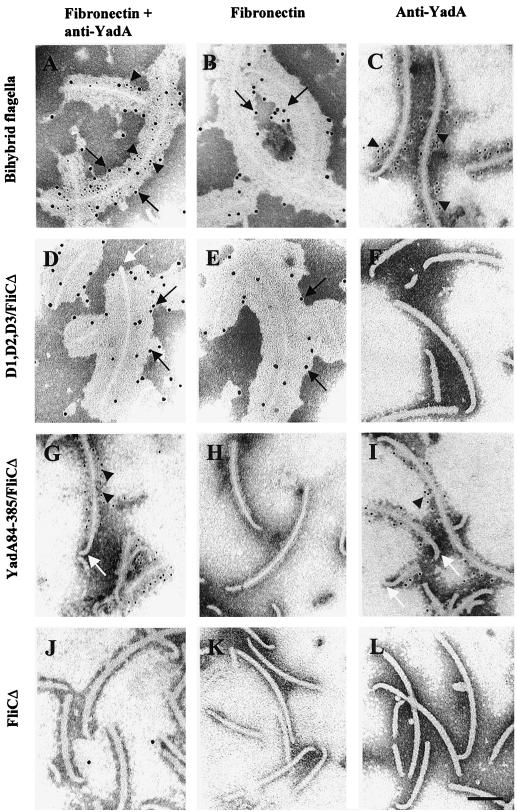
We used immunoelectron microscopy (IEM) as detailed recently (25) to analyze whether the two inserts were expressed along the same flagellar filament. Bacteria were immobilized onto copper grids coated with Pioloform and carbon and, to detect the YadA fragment, were left to react with the diluted monoclonal anti-YadA antibody 2G12 (25) for 1 h and 30 min at 20°C. The grids were washed, and bound antibodies were detected with secondary antibodies conjugated to colloidal gold particles with a diameter of 5 nm (Amersham, Little Chalfont, England). To detect expression of the D repeats, soluble human plasma fibronectin (100 μg/ml in 1% bovine serum albumin–phosphate-buffered saline [BSA-PBS]; Becton Dickinson, Bedford, Mass.) was added onto the grid and allowed to react with the flagella for 1 h at 20°C. After washing, bound fibronectin was detected with polyclonal antifibronectin antibodies (Chemicon, Temecula, Calif.) and Auroprobe EM protein A conjugate with gold particles 10 nm in diameter (Amersham). Control grids were prepared by omitting one reagent (anti-YadA antibodies, gold-conjugated secondary antibodies, fibronectin, antifibronectin antibodies, or gold-conjugated protein A) at a time. Bacteria were negatively stained by 1% potassium tungstic acid, pH 7.0, and the grids were examined in a Jeol 1200-EX transmission electron microscope at an operating voltage of 60 kV. The results of the IEM are shown in Fig. 2. Bihybrid flagellar filaments (Fig. 2A to C) bound anti-YadA antibodies as well as soluble fibronectin, and double staining of the flagella revealed both small and large immunogold particles along single flagellar filaments (Fig. 2A). The D1,D2,D3/FliCΔ hybrid flagella (Fig. 2D to F) bound fibronectin but not anti-YadA antibodies, and the YadA84-385/FliCΔ flagella (Fig. 2G to I) reacted with the anti-YadA antibodies. Binding of fibronectin to D repeat-containing flagella is seen as massive deposition of antifibronectin antibodies on the flagella. Control flagella lacking inserts (Fig. 2J to L) did not interact with soluble fibronectin or anti-YadA monoclonal antibodies. No immunostaining was observed in control samples lacking one of the reagents in the mixture (data not shown). The IEM results showed that both hybrid flagellins are incorporated into the same filaments.
FIG. 2.
Immunoelectron microscopy of hybrid flagella. The flagella were bihybrid flagella from E. coli BFS1 (A to C), the D1,D2,D3/FliCΔ flagella (D to F), the YadA84-385/FliCΔ flagella (G to I), and the FliCΔ flagella lacking an insert (J to L). Flagella were stained with monoclonal anti-YadA antibodies and with gold-conjugated secondary antibodies (5 nm in diameter) in C, F, I, and L; or with fibronectin, antifibronectin, and protein A-gold (10 nm in diameter) in B, E, H, and K. In A, D, G, and J, flagella double stained with both procedures are shown. Arrowheads indicate binding of anti-YadA antibodies, as visualized with 5-nm gold particles; black arrows indicate binding of fibronectin, as visualized with 10-nm gold particles; and white arrows indicate flagellar hooks. Size bar, 100 nm.
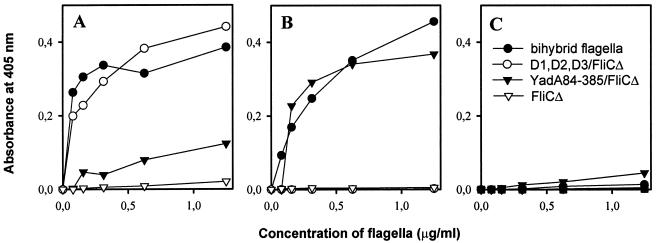
We earlier showed that the D1,D2,D3/FliCΔ hybrids specifically bind to immobilized or cell-bound fibronectin and that the YadA84-385/FliCΔ flagella recognize collagens (25). To assess the correct expression of the inserts in the BFS1 flagella, we tested binding of the bihybrid flagellar filaments by enzyme-linked immunosorbent assay (ELISA) technology, essentially as described previously (25). As the ELISA was based on immunological detection of flagella with anti-H7 flagellum antibodies, we first determined the reactivity of the chimeric flagella with the anti-H7 flagellum antibodies. Purified flagella were immobilized onto polystyrene 96-well microtiter plates (Nunc, Roskilde, Denmark) at a concentration of 5 μg of FliC/ml, anti-H7 flagellum antibodies (25) were added, and bound antibodies were detected with alkaline phosphatase-conjugated secondary antibodies. After addition of p-nitrophenyl phosphate substrate (Sigma), the absorbance at 405 nm was measured in a Multiscan Titertek recorder (Eflab, Helsinki, Finland). No significant differences were detected in the reactivity of the flagellar constructs with the anti-H7 flagellum antiserum (data not shown). To analyze the binding of the flagella to the target proteins, microtiter wells were coated with purified fibronectin and type IV collagen (Sigma) at a concentration of 2 pmol/well as described earlier (23), and fetuin (Sigma) was immobilized at a concentration of 25 μg/ml. After quenching and washing, hybrid flagella were added at a concentration of 0 to 1.25 μg of FliC/ml in 0.1% BSA-PBS, and 2 h later the wells were washed with PBS. Bound flagella were detected with polyclonal anti-H7 flagellum antibodies as described above. Bihybrid flagella bound to immobilized fibronectin and type IV collagen but not to fetuin, and the binding was dose dependent and saturable (Fig. 3). Flagella carrying D repeats bound to fibronectin. The YadA84-385/FliCΔ flagella bound to collagen as efficiently as did the bihybrid flagella (Fig. 3B), and a weak binding to immobilized fibronectin was also detected (Fig. 3A). Flagella lacking inserts did not bind to fibronectin or collagen, and none of the flagellar constructs bound to fetuin (Fig. 3). The weak binding of the YadA84-385/FliCΔ flagella to immobilized fibronectin is in accordance with the finding by Tamm and coworkers (21) that YadA binds strongly to laminin and collagens and only weakly to immobilized fibronectin. YadA does not bind to soluble fibronectin (22), which explains why YadA84-385/FliCΔ flagella did not bind to fibronectin in the IEM analysis.
FIG. 3.
Binding of hybrid flagella to proteins immobilized on plastic was determined by ELISA using anti-H7 flagellum antibodies and secondary antibodies. Binding in increasing concentrations (FliC at 0 to 1.25 μg/ml) of bihybrid flagella from E. coli BFS1 (—●—) and D1,D2,D3/FliCΔ (—○—), YadA84-385/FliCΔ (—▾—), and FliCΔ (—▿—) flagella to human plasma fibronectin (A), to type IV collagen (B) immobilized at a constant concentration of 2 pmol per well, and to fetuin (25 μg/ml) (C). Note the dose-dependent, saturable binding of bihybrid flagella to fibronectin as well as to type IV collagen.
Our results showed that the hybrid flagellins were expressed and polymerized in the same filament with equal frequency. Earlier studies have shown that up to 187-mer deletions and up to 36-mer insertions in the variable region of FliC can be constructed without loss of secretion of flagellin; such manipulations, however, frequently impair filament assembly (9, 11, 26) as well as function. Yoshioka and coworkers (26) described spontaneous, 89- and 97-mer deletions in the FliC of Salmonella typhimurium that affected the net charge of the FliC molecule and thereby the filament structure. It was concluded that electrostatic repulsive force between FliC subunits became weaker and destabilized the structure of the flagellar filament. The two FliC hybrids used in this study differed in size by 18 kDa from each other and by 15 and 33 kDa from FliCΔ, and the isoelectric points of the inserts, pI 4.4 (D repeats) and 8.6 (YadA84-385), differed from the pI 5.5 of the fragment deleted in FliCΔ. This fragment contains 8.6% positively charged and 10% negatively charged amino acid residues, whereas the amount of positively and negatively charged residues range from 4 to 13% and 4 to 24% in the foreign peptides that we have successfully displayed as fusions to FliCΔ. These results indicate that substantial differences in size, pI, and charge of the variable domain in FliC are tolerated in flagellar polymerization. This is an obvious advantage of the flagellum-based display system. The M13 phage display technology is based on fusions of foreign peptides to the major pVIII or the minor pIII coat protein of the filamentous bacteriophage. The use of pVIII as a carrier facilitates expression of up to 2,700 hybrid peptides on the phage surface, but the biotechnological applications of the method are limited by the size and sequence of the foreign peptides that are tolerated in pVIII. Inserts over 8 or 9 amino acids in length significantly hamper the assembly of the phage particle; the molecular basis for the variable sequence tolerance in pVIII is not well known but relies, apparently, upon the net charge as well as the conformation of the inserted peptide (15, 17). The host cell-binding protein pIII of phage M13 tolerates large inserts but is only expressed in five copies at one end of the phage, and large peptides in pIII also significantly decrease phage infectivity (17). The display of foreign epitopes in tobacco mosaic virus coat protein is affected by the charge as well as the pI of the inserted epitope; the pI of the insert must be near or equal to that of wild-type coat protein, and positively charged epitopes are only poorly tolerated (2). The inability of bacteria to form disulfide bonds in flagellins (25, 26) remains a limitation of the FliC display systems.
Bihybrid flagella can be used to present simultaneously, in the same flagellar filament, two different antigenic epitopes, thus facilitating immunization against two epitopes using a single type of antigen molecule. The technique offers a competitive tool for basic research as well as for a range of biotechnological applications. Multihybrid surface display systems can be applied in, e.g., construction of multivalent live bacterial vaccine strains or targeted effector bacteria or in histological localization and quantitation of specific tissue domains for diagnostic purposes. Construction and purification of hybrid flagella are relatively uncomplicated, and we are currently assessing the efficiency of multihybrid flagella in the production of serotype-specific antibodies for virus diagnostics.
Acknowledgments
We thank Mikael Skurnik for donating anti-YadA antibodies and Raili Lameranta, Anne Ikäheimonen, and Lena Blomqvist for skilled technical assistance. Electron microscopy was performed at the Electron Microscopy Unit, Institute of Biotechnology, University of Helsinki.
This study was supported by the Technology Development Centre Finland, the Academy of Finland (project numbers 42103, 42107, 44168, and 44600), and the University of Helsinki.
REFERENCES
- 1.Bäckström M, Lebens M, Schoedel F, Holmgren J. Insertion of a HIV-1-neutralizing epitope in a surface-exposed internal region of the cholera toxin B-subunit. Gene. 1994;149:211–217. doi: 10.1016/0378-1119(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 2.Bendahmane M, Koo M, Karrer E, Beachy R N. Display of epitopes on the surface of tobacco mosaic virus: impact of charge and isoelectric point of the epitope on virus-host interactions. J Mol Biol. 1999;290:9–20. doi: 10.1006/jmbi.1999.2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betton J-M, Jacob J P, Hofnung M, Broome-Smith J K. Creating a bifunctional protein by insertion of β-lactamase into the maltodextrin-binding protein. Nat Biotechnol. 1997;15:1276–1279. doi: 10.1038/nbt1197-1276. [DOI] [PubMed] [Google Scholar]
- 4.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 5.Fernández L A, Berenguer J. Secretion and assembly of regular surface structures in Gram-negative bacteria. FEMS Microbiol Rev. 2000;24:21–44. doi: 10.1111/j.1574-6976.2000.tb00531.x. [DOI] [PubMed] [Google Scholar]
- 6.Flock J-I, Fröman G, Jönsson K, Guss B, Signäs C, Nilsson B, Raucci C, Höök M, Wadström T, Lindberg M. Cloning and expression of the gene for a fibronectin-binding protein from Staphylococcus aureus. EMBO J. 1987;6:2351–2357. doi: 10.1002/j.1460-2075.1987.tb02511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frankel A E, Burbage C, Fu T, Tagge E, Chandler J, Willingham M. Characterization of a ricin fusion toxin targeted to the interleukin-2 receptor. Protein Eng. 1996;9:913–919. doi: 10.1093/protein/9.10.913. [DOI] [PubMed] [Google Scholar]
- 8.Georgiou G, Stathopoulos C, Daugherty P S, Nayak A R, Iverson B L, Curtiss R., III Display of heterologous proteins on the surface of microorganisms: from the screening of combinatorial libraries to live recombinant vaccines. Nat Biotechnol. 1997;15:29–33. doi: 10.1038/nbt0197-29. [DOI] [PubMed] [Google Scholar]
- 9.He X-S, Rivkina M, Stocker B A D, Robinson W S. Hypervariable region IV of Salmonella gene fliCd encodes a dominant surface epitope and stabilizing factor for functional flagella. J Bacteriol. 1994;176:2406–2414. doi: 10.1128/jb.176.8.2406-2414.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwajima G. Flagellin domain that affects H antigenicity of Escherichia coli K-12. J Bacteriol. 1988;170:485–488. doi: 10.1128/jb.170.1.485-488.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwajima G. Construction of a minimum-size functional flagellin of Escherichia coli. J Bacteriol. 1988;170:3305–3309. doi: 10.1128/jb.170.7.3305-3309.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebens M, Shahabi V, Bäckström M, Houze T, Lindblad M, Holmgren J. Synthesis of hybrid molecules between heat-labile enterotoxin and cholera toxin B subunits: potential for use in a broad-spectrum vaccine. Infect Immun. 1996;64:2144–2150. doi: 10.1128/iai.64.6.2144-2150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loregian A, Papini E, Satin B, Marsden H S, Hirst T R, Palù G. Intranuclear delivery of an antiviral peptide mediated by the B subunit of Escherichia coli heat-labile enterotoxin. Proc Natl Acad Sci USA. 1999;96:5221–5226. doi: 10.1073/pnas.96.9.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Z, Murray K S, Van Cleave V, LaVallie E R, Stahl M L, McCoy M. Expression of thioredoxin random peptide libraries on the Escherichia coli cell surface as functional fusions to flagellin: a system designed for exploring protein-protein interactions. Biotechnology. 1995;13:366–372. doi: 10.1038/nbt0495-366. [DOI] [PubMed] [Google Scholar]
- 15.Malik P, Perham R N. Simultaneous display of different peptides on the surface of filamentous bacteriophage. Nucleic Acids Res. 1996;25:915–916. doi: 10.1093/nar/25.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namba K, Yamashita I, Vonderviszt F. Structure of the core and central channel of bacterial flagella. Nature. 1989;342:648–654. doi: 10.1038/342648a0. [DOI] [PubMed] [Google Scholar]
- 17.Rodi D J, Makowski L. Phage-display technology—finding a needle in a vast molecular haystack. Curr Opin Biotechnol. 1999;10:87–93. doi: 10.1016/s0958-1669(99)80016-0. [DOI] [PubMed] [Google Scholar]
- 18.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Signäs C, Raucci G, Jönsson K, Lindgren P-E, Ananantharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ståhl S, Uhlén M. Bacterial surface display: trends and progress. Trends Biotechnol. 1997;15:185–192. doi: 10.1016/S0167-7799(97)01034-2. [DOI] [PubMed] [Google Scholar]
- 21.Tamm A, Tarkkanen A-M, Korhonen T K, Kuusela P, Toivanen P, Skurnik M. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol Microbiol. 1993;10:995–1011. doi: 10.1111/j.1365-2958.1993.tb00971.x. [DOI] [PubMed] [Google Scholar]
- 22.Tertti R, Skurnik M, Vartio T, Kuusela P. Adhesion protein YadA of Yersinia species mediates binding of bacteria to fibronectin. Infect Immun. 1992;60:3021–3024. doi: 10.1128/iai.60.7.3021-3024.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westerlund B, Kuusela P, Risteli J, Risteli L, Vartio T, Rauvala H, Virkola R, Korhonen T K. The O75X adhesin of uropathogenic Escherichia coli is a type IV collagen-binding protein. Mol Microbiol. 1989;3:329–337. doi: 10.1111/j.1365-2958.1989.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 24.Westerlund-Wikström, B. Peptide display on bacterial flagella: principles and applications. Int. J. Med. Microbiol., in press. [DOI] [PubMed]
- 25.Westerlund-Wikström B, Tanskanen J, Virkola R, Hacker J, Lindberg M, Skurnik M, Korhonen T K. Functional expression of adhesive peptides as fusions to Escherichia coli flagellin. Protein Eng. 1997;10:1319–1326. doi: 10.1093/protein/10.11.1319. [DOI] [PubMed] [Google Scholar]
- 26.Yoshioka K, Aizawa S-I, Yamaguchi S. Flagellar filament structure and cell motility of Salmonella typhimurium mutants lacking part of the outer domain of flagellin. J Bacteriol. 1995;177:1090–1093. doi: 10.1128/jb.177.4.1090-1093.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]