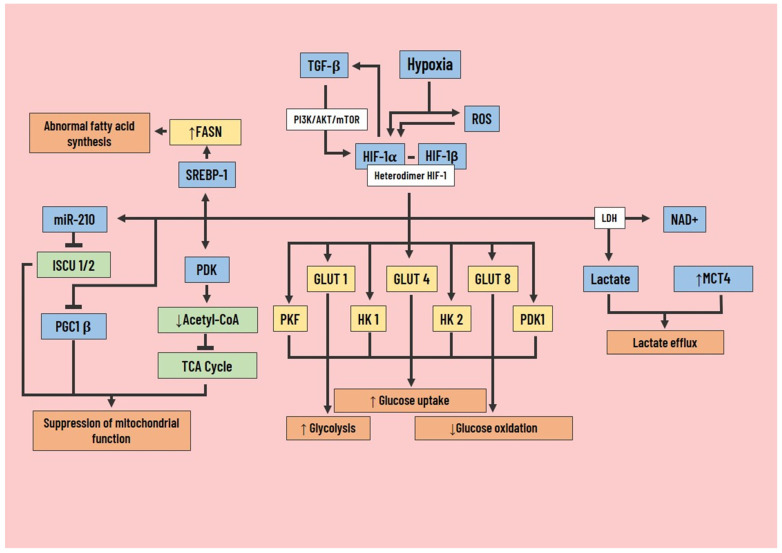
Figure 4.
Role of hypoxia inducible factor-1 in cancer cell metabolic reprogramming. Hypoxia modifies cell metabolism through the expression of HIF-1α, which influences tumor angiogenesis, cancer cell migration, invasion, and glycolytic metabolism. In addition to this, HIF-1α can be activated by TGF-β action via the PI3K/AKT/mTOR pathway, and at the same time, HIF-1α can activate TGF-β in a positive feedback loop. Among the most important metabolic changes produced by HIF-1α are increased glucose uptake, glycolysis, and decreased glucose oxidation through the induction of GLUT 1/4/8, PKF, HK 1/2, and PGK1. There is also an increase in lactate and NAD+ production through LDH. Together, an increase in MCT4 expression contributes to lactate efflux. Another effect of HIF-1α expression is the suppression of mitochondrial function, resulting from inactivation of the TCA cycle, inhibition of PGC1 β, and repression of ISCU 1/2 by miR-210. Finally, HIF-1α promotes abnormal fatty acid synthesis through overexpression of FASN, which is mediated by increased SREBP-1 activation. HIF-1α, hypoxia-inducible factor-1; TGF-β, transforming growth factor beta; PI3K, phosphoinositol 3-kinase; LDH, lactate dehydrogenase; NAD, nicotinamide adenine dinucleotide; MCT4, monocarboxylate transporter 4; GLUT, glucose transporter; PKF, phosphofructokinase; PGK1, phosphoglycerate kinase 1; HK, hexokinase; PDK, phosphoinositide-dependent kinase; TCA, tricarboxylic acid; PGC1 β, peroxisome proliferator-activated receptor-gamma coactivator-1 beta; miR-210, microRNA-210; ISCU, iron-sulfur group; SREBP-1, sterol regulatory element binding protein 1; FASN, fatty acid synthase; ↑, increase; ↓, decrease.