Abstract
• PURPOSE:
To examine the association between cognitive dementia and retinal vascular occlusions.
• DESIGN:
A retrospective, cross-sectional study.
• METHODS:
Single-institution study population: we reviewed the electronic medical records of 37,208 individuals older than 65 years of age who were evaluated by an ophthalmologist or an optometrist and who also had a medical visit to our institution over a 6-year period. Individuals with and without retinal vascular occlusions were identified by International Classification of Diseases, version 10 (ICD-10) diagnostic codes.
• MAIN OUTCOME:
we analyzed the association between dementia and retinal vascular occlusions after adjusting for covariates which included age, sex, stroke, diabetes mellitus, and hypertension using multiple logistic regression analyses.
• RESULTS:
Compared to subjects without retinal vascular occlusions, those with retinal vascular occlusions had a higher prevalence of dementia (6.7% vs. 9.3%, respectively; P < .001). After adjusting for either age or stroke, there were no significant associations between retinal vascular occlusions and dementia.
• CONCLUSIONS:
Individuals with retinal vascular occlusions have a higher prevalence of dementia. However, this association is secondary to shared underlying risk factors in this population, such as older age and stroke.
Dementia is a leading cause of morbidity and mortality in the elderly, and the increasing worldwide burden of dementia has sparked interest in identifying risk factors related to dementia.1–8 Retinal imaging has demonstrated that retinal vascular changes are detectable in individuals with dementia9–11 and that retinal vein occlusions have been recently shown to confer an increased risk of dementia.3 However, individuals with dementia and retinal vascular occlusions share common risk factors such as older age, cardiovascular disease, and stroke.1, 4–6, 12–31 What is unknown is whether retinal vascular occlusions are associated with increased risk of dementia, independent of these common risk factors.
METHODS
This was a retrospective, cross-sectional study conducted in accordance with the Health Insurance Portability and Accountability Act. Internal Review Board (IRB) approval was obtained from the University of California San Diego Health System.
We reviewed the records of 37,208 individuals older than 65 years of age, who were evaluated by an ophthalmologist or an optometrist and who also had a medical visit to our institution between January 1, 2015, and December 31, 2020. Individuals with retinal vascular occlusions were identified through International Classification of Diseases, edition 10 (ICD-10) diagnostic codes. In addition, we identified individuals with dementia (vascular dementia, ICD-10 F01; dementia in other diseases classified elsewhere, ICD-10 F02; unspecified dementia, ICD-10 F03; Alzheimer’s disease, ICD-10 G30), stroke (cerebral infarction, ICD-10 I63), essential hypertension (ICD-10 I10) and type 2 diabetes mellitus (ICD-10 E11). Baseline characteristics of subjects with and without retinal vascular occlusions were compared using Student’s t-test and Pearson’s χ2 test, where applicable. Multivariate logistic regression models were used to analyze the association between retinal vascular occlusions and dementia. Odds ratios (OR) with 95% confidence intervals (CI) were calculated. Statistical analyses and graphs were generated using R version 4.0.3 software (R Foundation, Vienna, Austria).
RESULTS
We identified 904 individuals with and 36,304 individuals without retinal vascular occlusions. Demographics and baseline characteristics of the study cohort are shown in Table 1. Individuals with retinal vascular occlusions were older than those without (79 vs. 76 years of age, respectively; P < .001), and the proportion of females was lower in the retinal vascular occlusion group (51.0 vs. 57.2%, respectively; P < .001). There were no significant differences in race between the groups (P = .62). Compared to individuals without, those with retinal vascular occlusions had a higher prevalence for dementia (6.7% vs. 9.3%, respectively; P = .003), stroke (7.3% vs. 18.1%, respectively; P < .001), hypertension (62.1% vs. 77.0%, respectively; P <.001) and diabetes mellitus (26.9% vs. 36.4%, respectively; P < .001).
TABLE 1.
Characteristics of the Study Cohorta
| Retinal Vascular Occlusions Present | Retinal Vascular Occlusions Absent | P Value | |
|---|---|---|---|
| Mean ± SD age, y | 79 ± 8 | 76 ± 8 | < .001 |
| Females | 461 (51.0) | 20,756 (57.2) | < .001 |
| Race | .62 | ||
| White | 603 (66.7) | 24,269 (66.8) | |
| Asian | 112 (12.4) | 5,011 (13.8) | |
| Black | 33 (3.7) | 1,067 (2.9) | |
| Native Hawaiian or Pacific Islander | 4 (0.4) | 154 (0.4) | |
| American Indian or Alaska Native | 3 (0.3) | 133 (0.4) | |
| Other | 154 (17.0) | 5,770 (15.9) | |
| Dementia | 84 (9.3) | 2,440 (6.7) | .003 |
| Stroke | 164 (18.1) | 2,645 (7.3) | < .001 |
| Hypertension | 696 (77.0) | 22,556 (62.1) | < .001 |
| Diabetes mellitus | 329 (36.4) | 9,768 (26.9) | < .001 |
Table values are mean ± SD and n (%).
Mean and standard deviations are reported for age. Percentages are reported for females, races, dementia, stroke, hypertension, and diabetes mellitus.
Given that dementia and retinal vascular occlusions share similar risk factors, we analyzed the association between dementia and retinal vascular occlusions after adjusting for each of the significant covariates including age, sex, stroke, hypertension, and diabetes mellitus by using multiple logistic regression analyses.
After we adjusted for sex, hypertension, or diabetes, or all 3 covariates, retinal vascular occlusions were still significantly associated with dementia, with OR of 1.42 (95% CI: 1.12–1.78], 1.27 (95% CI: 1.01–1.59), 1.36 (95% CI: 1.08–1.70), and 1.27 (95% CI: 1.00–1.58), respectively (Table 2). However, after we adjusted for either age or stroke there was no significant association between retinal vascular occlusions and dementia, OR 1.10 (95% CI: 0.86–1.38) and 1.12 (95% CI: 0.88–1.41), respectively (Table 2).
TABLE 2.
OR for Presence of Dementia in Individuals with Retinal Vascular Occlusions a
| Age groups (n) | Covariates adjusted for | OR | 95% CI | P Value |
|---|---|---|---|---|
| ≥65 y (37,208) | None | 1.42 | 1.12–1.78 | .003 |
| Sex | 1.42 | 1.12–1.78 | .003 | |
| Hypertension | 1.27 | 1.01–1.59 | .04 | |
| Diabetes mellitus | 1.36 | 1.08–1.70 | .008 | |
| Age | 1.10 | 0.86–1.38 | .44 | |
| Stroke | 1.12 | 0.88–1.41 | .34 | |
| Sex, hypertension and diabetes mellitus | 1.27 | 1.00–1.58 | .04 | |
| Age and stroke | 0.94 | 0.73–1.18 | .59 | |
| 65–74 y (18,382) | None | 0.93 | 0.39–1.82 | .85 |
| 75–84 y (12,777) | None | 1.01 | 0.66–1.48 | .96 |
| ≥85 y (6,049) | None | 1.20 | 0.86–1.63 | .27 |
OR: Odds ratio; CI: confidence interval.
Odds ratios are shown for the presence of dementia in individuals with retinal vascular occlusions, after adjusting for different covariates using multivariable logistic regression models.
We also analyzed the following associations between dementia and retinal vascular occlusions in different age groups: 65–74 years of age (n = 18,382), 75–84 years of age (n = 12,777), and ≥85 years of age (n = 6,049). Within each age group, there were no statistically significant differences in age between individuals with and without retinal vascular occlusions. There were no significant associations between retinal vascular occlusions and dementia in any of the 3 age groups. The OR were 0.93 (95% CI: 0.39–1.82) for individuals 65–74 years of age; 1.01 (95% CI: 0.66–1.48) for individuals 75–84 years of age; and 1.20 (95% CI: 0.86–1.63) for individuals ≥85 years of age, respectively (Table 2).
Finally, we analyzed the association between dementia and all of the covariates including retinal vascular occlusions. Stroke, older age, diabetes, and hypertension were each associated with dementia, whereas retinal vascular occlusions and female sex were not significantly associated with dementia (Figure). For retinal vascular occlusions, the OR of dementia was 0.88 (95% CI: 0.69–1.12).
FIGURE.
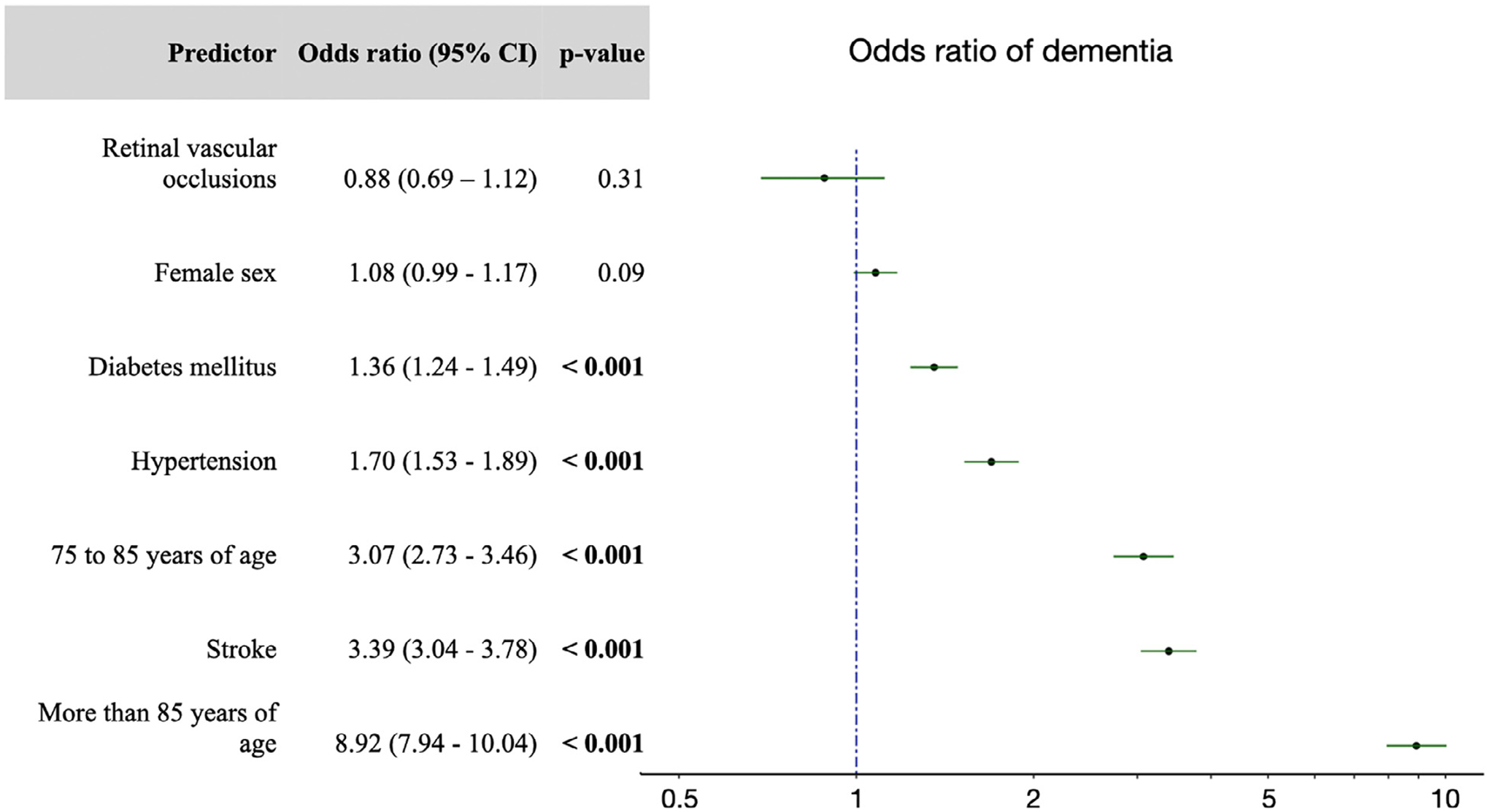
Odds ratios for dementia. A multivariate logistic regression model was used to identify the presence of dementia. Covariates included stroke, female sex, hypertension, diabetes mellitus, and age groups 75–84 years of age and ≥85 years of age, with a reference age group of 65–74 years of age. Error bars represent 95% confidence intervals. The dotted line indicates an odds ratio of 1.
DISCUSSION
We examined the association between the retinal vascular occlusions and dementia in a retrospective cohort of individuals older than 65 years of age. We found that individuals with retinal vascular occlusions have increased odds of having dementia, in line with a recently published report.3 However, after we adjusted for age or stroke, there were no significant associations between retinal vascular occlusions and dementia.
The major strengths of our study are the large sample size and adjustment for confounding risk factors for dementia. Limitations of this study include the inherent weakness of retrospective study design. Because we relied on diagnostic billing codes, there was a possibility for misclassification of diagnoses. Additionally, dementia is often under-diagnosed in the clinical setting. We also did not examine the association between retinal vascular occlusions and individual subtypes of dementia such as Alzheimer’s disease, vascular dementia, and dementia with Lewy bodies. Additionally, the contributions of other factors associated with cognitive decline, including genetic risk factors and socioeconomic status, were not assessed. Finally, the cohort was composed mainly of Whites (66.8%) and Asians (12%), which limited the generalizability of our findings to other populations.
In conclusion, we demonstrate that the increased association between retinal vascular occlusions and dementia is secondary to shared underlying risk factors in this population, mainly older age and presence of stroke.
FUNDING/SUPPORT:
This work was supported by Department of Veterans’ Affairs Clinical Sciences Research and Development Service merit award 1I01CX001842 (to Katherine J. Bangen) and National Institute on Aging/National Institutes of Health grant R01 AG063782 (to Katherine J. Bangen).
Footnotes
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
FINANCIAL DISCLOSURES: None.
REFERENCES
- 1.Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: a systematic review and meta-analysis. Alzheimers Dement. 2018;14: 1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rensma SP, van Sloten TT, Launer LJ, Stehouwer CDA. Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:164–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nam GE, Han K, Park SH, Cho KH, Song SJ. Retinal vein occlusion and the risk of dementia: a nationwide cohort study. Am J Ophthalmol. 2021;221:181–189. [DOI] [PubMed] [Google Scholar]
- 4.Zlokovic BV, Gottesman RF, Bernstein KE, et al. Vascular contributions to cognitive impairment and dementia (VCID): a report from the 2018 National Heart, Lung, and Blood Institute and National Institute of Neurological Disorders and Stroke Workshop. Alzheimers Dement. 2020;16(12):1714–1733. doi: 10.1002/alz.12157. [DOI] [PubMed] [Google Scholar]
- 5.Surawan J, Areemit S, Tiamkao S, Sirithanawuthichai T, Saensak S. Risk factors associated with post-stroke dementia: a systematic review and meta-analysis. Neurol Int. 2017;9:7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Justin BN, Turek M, Hakim AM. Heart disease as a risk factor for dementia. Clin Epidemiol. 2013;5:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalaria RN, Maestre GE, Arizaga R, et al. Alzheimer’s disease and vascular dementia in developing countries: prevalence, management, and risk factors. Lancet Neurol. 2008;7:812–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology. 2005;64:277–281. [DOI] [PubMed] [Google Scholar]
- 9.de Jong FJ, Schrijvers EM, Ikram MK, et al. Retinal vascular caliber and risk of dementia: the Rotterdam study. Neurology. 2011;76:816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang YS, Zhou N, Knoll BM, et al. Parafoveal vessel loss and correlation between peripapillary vessel density and cognitive performance in amnestic mild cognitive impairment and early Alzheimer’s disease on optical coherence tomography angiography. PLoS One. 2019;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gupta VB, Chitranshi N, den Haan J, et al. Retinal changes in Alzheimer’s disease- integrated prospects of imaging, functional and molecular advances. Prog Retin Eye Res. 2020. [DOI] [PubMed] [Google Scholar]
- 12.Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye (Lond). 2016;30:1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christiansen CB, Lip GY, Lamberts M, Gislason G, Torp–Pedersen C, Olesen JB. Retinal vein and artery occlusions: a risk factor for stroke in atrial fibrillation. J Thromb Haemost. 2013;11:1485–1492. [DOI] [PubMed] [Google Scholar]
- 14.Plunkett O, Lip PL, Lip GY. Atrial fibrillation and retinal vein or artery occlusion: looking beyond the eye. Br J Ophthalmol. 2014;98:1141–1143. [DOI] [PubMed] [Google Scholar]
- 15.Park SJ, Choi NK, Yang BR, Park KH, Woo SJ. Risk of stroke in retinal vein occlusion. Neurology. 2015;85:1578–1584. [DOI] [PubMed] [Google Scholar]
- 16.Ponto KA, Elbaz H, Peto T, et al. Prevalence and risk factors of retinal vein occlusion: the Gutenberg Health Study. J Thromb Haemost. 2015;13:1254–1263. [DOI] [PubMed] [Google Scholar]
- 17.Rim TH, Han J, Choi YS, et al. Retinal artery occlusion and the risk of stroke development: twelve-year nationwide cohort study. Stroke. 2016;47:376–382. [DOI] [PubMed] [Google Scholar]
- 18.Rim TH, Oh J, Lee CS, Lee SC, Kang SM, Kim SS. Evaluation of the association between retinal vein occlusion and the risk of atrial fibrillation development: a 12–year, retrospective nationwide cohort study. Sci Rep. 2016;6:34708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisa Gracia M, Córdoba Alonso A, Hernández Hernández JL, Pérez Montes R, Napal Lecumberri JJ. Cardiovascular risk factors, nonvalvular atrial fibrillation and retinal vein occlusion. Rev Clin Esp. 2017;217:188–192. [DOI] [PubMed] [Google Scholar]
- 20.Bakhoum MF, Freund KB, Dolz-Marco R, et al. Paracentral acute middle maculopathy and the ischemic cascade associated with retinal vascular occlusion. Am J Ophthalmol. 2018;195:143–153. [DOI] [PubMed] [Google Scholar]
- 21.Chen YY, Yen YF, Lin JX, et al. Risk of ischemic stroke, hemorrhagic stroke, and all-cause mortality in retinal vein occlusion: a nationwide population-based cohort study. J Ophthalmol. 2018;2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Christiansen CB, Torp-Pedersen C, Olesen JB, et al. Risk of incident atrial fibrillation in patients presenting with retinal artery or vein occlusion: a nationwide cohort study. BMC Cardiovasc Disord. 2018;18:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol. 2018;196: 96–100. [DOI] [PubMed] [Google Scholar]
- 24.Fallico M, Lotery AJ, Longo A, et al. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye (Lond). 2020;34(4):683–689. doi: 10.1038/s41433-019-0576-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kewcharoen J, Tom ES, Wiboonchutikula C, et al. Prevalence of atrial fibrillation in patients with retinal vessel occlusion and its association: a systematic review and meta-analysis. Curr Eye Res. 2019;44:1337–1344. [DOI] [PubMed] [Google Scholar]
- 26.Ponto KA, Scharrer I, Binder H, et al. Hypertension and multiple cardiovascular risk factors increase the risk for retinal vein occlusions: results from the Gutenberg Retinal Vein Occlusion Study. J Hypertens. 2019;37:1372–1383. [DOI] [PubMed] [Google Scholar]
- 27.Wu CY, Riangwiwat T, Limpruttidham N, Rattanawong P, Rosen RB, Deobhakta A. Association of retinal vein occlusion with cardiovascular events and mortality: a systematic review and meta-analysis. Retina. 2019;39:1635–1645. [DOI] [PubMed] [Google Scholar]
- 28.Fallico M, Lotery AJ, Longo A, et al. Risk of acute stroke in patients with retinal artery occlusion: a systematic review and meta-analysis. Eye (Lond). 2020;34:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leisser C, Findl O. Rate of strokes 1 year after retinal artery occlusion with analysis of risk groups. Eur J Ophthalmol. 2020;30:360–362. [DOI] [PubMed] [Google Scholar]
- 30.Schorr EM, Rossi KC, Stein LK, Park BL, Tuhrim S, Dhamoon MS. Characteristics and outcomes of retinal artery occlusion: nationally representative data. Stroke. 2020;51:800–807. [DOI] [PubMed] [Google Scholar]
- 31.Xiao YY, Wei WB, Wang YX, et al. Correlation of the history of stroke and the retinal artery occlusion: a nested case-control study. Int J Ophthalmol. 2020;13:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]


