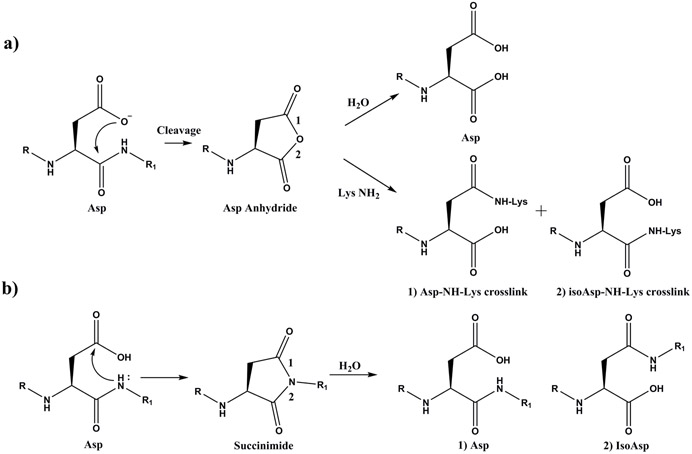
Scheme 1. Asp residues in proteins can breakdown in two ways: cleavage or racemisation/isomerisation.
Pathway a) Cleavage. Asp residues can undergo spontaneous cyclisation forming a cyclic anhydride intermediate. Detailed analyses indicate that the mechanism probably involves attack of the ionized side chain carboxyl on a protonated carbonyl of the peptide bond [38]. The cyclic anhydride can hydrolyse, or can react with an amine group such as that of a Lys residue, forming an Asp-Lys crosslink. The ε-amino group of Lys can potentially attack either carbonyl of the anhydride leading to two isomeric Asp-Lys crosslinks as shown.
Pathway b) Racemisation/isomerisation. Formation of a succinimide can occur by attack of the adjacent peptide bond NH group on the side chain carboxyl group of Asp. The succinimide can isomerise and hydrolyse to yield four different Asp isomers: L-Asp, D- Asp, L-isoAsp, and D-isoAsp [22].