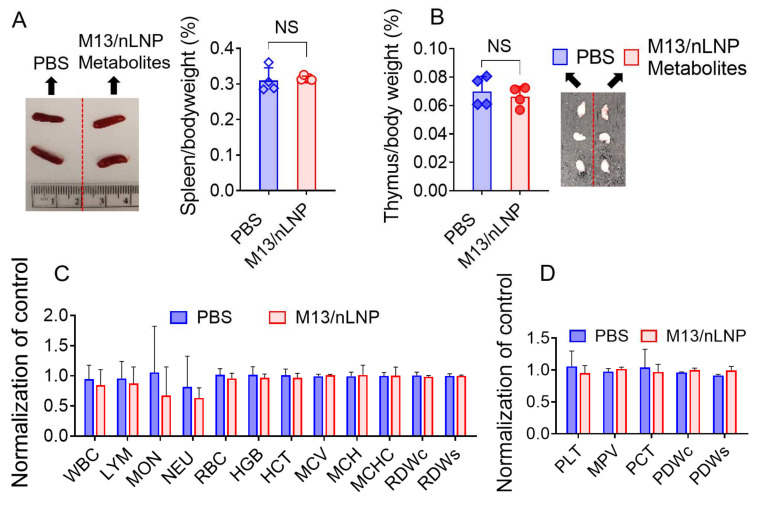
Figure 7.
Safety evaluation for M13/nLNP-incubated microbiota-secreted metabolites in IL-10 KO mice. (A) Representative spleen picture and spleen-to-bodyweight ratio for M13/nLNP-incubated microbiota-secreted metabolite-treated and control mice; (B) thymus-to-bodyweight ratio and representative thymus pictures from the two groups; (C) blood was collected from the retro-orbital sinus, and hematological analyses were performed using an automatic hematology analyzer (VetScan HM5; Abaxis, CA, USA). The following hematologic parameters are shown: WBC—total white blood cells; LYM—lymphocytes; MON—monocytes; NEU—neutrophils; RBC—red blood cells; HGB—hemoglobin; HCT—hematocrit; MCV—mean corpuscular volume; MCH—hemoglobin amount per red blood cell; MCHC—mean corpuscular hemoglobin concentration; RDWc—red cell distribution width cv; RDWs—red cell distribution width sd; (n = 4). (D) PLT—platelets; MPV—mean platelet volume; PCT—procalcitonin; PDWc—platelet distribution width cv; PDWs—platelet distribution width sd. (n = 4).