Table 2.
Summary of characteristics and findings of studies that examined the effect of non-pharmacological self-management interventions on chemotherapy-induced peripheral neuropathy symptoms and related outcomes in people with advanced cancer.
| Study and Population Characteristics | Intervention Characteristics | Findings | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CIPN | CIPN-Related Outcomes | |||||||||||||||||||
| Citation and Country | Population | Cancer | CTX | Intervention | Control | Tool and Time Point | Incidence | Severity | Quality of Life | Physical function | Pain | Sleep | Fatigue | GI symptoms | Nutrition status | Psychological | Social | Treatment | Financial | Adverse events |
| Physical exercise | ||||||||||||||||||||
| Henke 2014 [31]; Germany |
N: 46 Age (yrs): NR Males: NR |
Type: lung Stage: ≥3 Existing CIPN: NR |
Type: Platinum-based Frequency: NR Duration: NR Status: ongoing |
Strategy: Strength and endurance training (n = 25) Regimen: 8 min endurance or 3 sets of 4 strength exercises daily Duration: 3 CTX cycles, from C1D1 |
Standard care (n = 21) | EORTC QLQ-LC13; pre and post (C3) | + | + | + | + | o | o | o | o | o | o | ||||
| Stuecher 2019 [32]; Germany | N: 44 Age (yrs): 67 ± 8 Males: 67% |
Type: gastrointestinal Stage: ≥3 Existing CIPN: no |
Type: NR Frequency: NR Duration: NR Status: ongoing |
Strategy: Walking (n = 22) Regimen: 150 min per wk Duration: 12 wks, from C1D1 |
Standard care (n = 22) | Tuning fork test; pre and post (6 and 12 wks) | o | + | + | o | ||||||||||
| Streckmann 2014 [33]; Germany | N: 61 Age (yrs): 46 (19–73) Males: 77% |
Type: lymphoma Stage: progressive Existing CIPN: NR |
Type: mixed Frequency: NR Duration: NR Status: ongoing |
Strategy: Strength, endurance, and sensorimotor training (n = 30) Regimen: 1 h session twice per wk Duration: 36 wks |
Standard care (n = 31) | Tuning fork test; pre and post (12, 24 and 36 wks) | + | o | + | o | + | + | + | + | + | o | ||||
| Zimmer 2018 [34]; Germany |
N: 30 Age (yrs): 50–81 Males: 70% |
Type: colorectal Stage: 4 Existing CIPN: NR |
Type: mixed Frequency: NR Duration: 2–3 cycles Status: ongoing and ceased |
Strategy: Strength, endurance, and sensorimotor training (n = 17) Regimen: 1 hr session twice per wk Duration: 8 wks |
Written exercise guidelines (n = 13) | FACT/GOG-NTX; pre and post (8 and 12 wks) | + | o | + | o | o | o | ||||||||
| Nutrition supplements | ||||||||||||||||||||
| Bradfield, 2015 [35]; USA | N: 200 Age (yrs): 9 ± 5 Males: 62% |
Type: lymphoma Stage: NR Existing CIPN: no |
Type: Vincristine Frequency: weekly Duration: ≥4 wks Status: ongoing |
Strategy: L-glutamic acid in capsule form, taken orally (n = 101) Regimen: 3 times daily, total 0.75–1.5 g per day Duration: 5 wks |
Placebo (n = 99) | mBPSPN; pre and post (5 wks) | o | |||||||||||||
| Howells, 2019 [36]; UK | N: 27 Age (yrs): 68 (53–78) Males: NR |
Type: colorectal Stage: metastatic Existing CIPN: no |
Type: 5FU and oxaliplatin Frequency: fortnightly Duration: ≤12 cycles Status: ongoing |
Strategy: Curcumin powder in capsule form, taken orally (n = 18) Regimen: 4 times daily, total 2 g per day Duration: duration of CTX (from 7 days before C1D1) |
Standard care (n = 9) | EORTC-QLQ-C30 and NCI-CTAE; pre and post | - | o | o | o | o | o | o | - | o | + | o | |||
| Sanchez-Lara, 2014 [37]; Mexico | N: 112 Age (yrs): 18–80 Males: 47% |
Type: NSCL Stage: ≥3 b Existing CIPN: NR |
Type: paclitaxel and cisplatin/carboplatin Frequency: every wks Duration: 2–6 cycles Status: ongoing |
Strategy: omega 3 (EPA)-enriched oral nutrition supplement + isocaloric diet (n = 54) Regimen: 2 237 mL drinks per day (provides 2.2 g EPA) Duration: 2 CTX cycles, from C1D1 |
Isocaloric diet (n = 58) | EORTC-QLQ-C30 and -LC13; pre and post (C1 and C2) | + | o | o | + | + | + | o | o | ||||||
| Wang, 2007 [38]; Taiwan |
N: 86 Age (yrs): 60% ≥50 Males: 65% |
Type: colorectal Stage: metastatic Existing CIPN: no |
Type: 5FU and oxaliplatin Frequency: every 4 wks Duration: NR Status: ongoing |
Strategy: Levo-Glutamine, taken orally (n = 42) Regimen: twice daily, total 30 g per day for 7 days every 2 wks Duration: 6 cycles, from C1D1 |
Standard care (n = 44) | NCI-CTCAE and Electro-physiological exam; pre and post (C2, C4 and C6) |
+ | + | + | o | ||||||||||
| Japanese herbal medicine | ||||||||||||||||||||
| Motoo 2020 [39]; Japan | N: 52 Age (yrs): 35–79 Males: 60% |
Type: colorectal Stage: 3 Existing CIPN: no |
Type: capecitabine and oxaliplatin Frequency: every 3 wks Duration: 8 cycles Status: ongoing |
Strategy: ninjin’yoeito powder 1, taken orally (n = 26) Regimen: 2–3 times daily, total 9 g per day Duration: 8 cycles, from C1D1 |
Standard care (n = 26) | NCI-CTCAE; pre and post (C1–C8) | + | o | o | o | + | o | ||||||||
| Niskioka, 2011 [40]; Japan | N: 45 Age (yrs): 48–80 Males: 49% |
Type: colorectal Stage: metastatic Existing CIPN: no |
Type: 5FU and oxaliplatin Frequency: every 2 wks Duration: 4–32 cycles Status: ongoing |
Strategy: Goshajinkigan 2, taken orally (n = 22) Regimen: 2–3 times daily, total 7.5 g per day Duration: entire CTX course (4–32 cycles), from C1D1 |
Standard care (n = 23) | DEB-NTC; pre and post (at each CTX cycle) | + | o | o | o | ||||||||||
| Oki, 2015 [41]; Japan | N: 186 Age (yrs): 61 ± 11 Males: 55% |
Type: colorectal Stage: 3 Existing CIPN: no |
Type: 5FU and oxaliplatin Frequency: every 2 wks Duration: 12 cycles Status: ongoing |
Strategy: Goshajinkigan 2, taken orally (n = 93) Regimen: daily with meals, total 7.5 g per day Duration: entire CTX course (12 cycles), from C1D1 |
Placebo (n = 93) | NCI-CTCAE and DEB-NTC; pre and post (at each CTX cycle) | - | o | o | + | o | |||||||||
| Technology-facilitated education for symptom self-management | ||||||||||||||||||||
| Given, 2008 [42]; USA | N: 47 Age (yrs): ≥21 Males: 0% |
Type: breast Stage: metastatic Existing CIPN: NR |
Type: mixed Frequency: NR Duration: NR Status: ongoing |
Strategy: Education for symptom self-management via automated telephone voice technology incorporating symptom monitoring (n = 24) Regimen: weekly phone calls for 4 wks, then at wk 6 and wk 8 Duration: 8 wks |
Cognitive behavioral nurse-administered telephone symptom management (n = 23) | 11-point Likert scale; pre and post (10 and 16 wks) | ? | ? | ? | ? | ? | ? | ? | |||||||
| Kim, 2018 [43]; Korea |
N: 76 Age (yrs): 51 ± 7 Males: 0% |
Type: breast Stage: 4 Existing CIPN: NR |
Type: mixed Frequency: NR Duration: NR Status: ongoing |
Strategy: Education for symptom self-management via a mobile phone game (n = 36) Regimen: >30 min per day, 3 times per wk Duration: 3 wks |
Symptom management booklet (n = 40) | NCI-CTCAE; pre and post (3 wks) | + | + | - | - | - | o | + | o | ||||||
1 Contains 12 crude Japanese herbs: Rehmannia root, Angelica root, Atractylodes rhizome, Poria Sclerotium, Ginseng, Cinnamon bark, Polygala root, Peony root, Citrus Unshiu peel, Atsragalus root, Glycyrrhiza, Schisandra fruit. 2 Contains 10 crude Japanese herbs: Rehmannia root, Achyranthes root, Cornus fruit, Dioscorea rhizome, Plantago seed, Alisma Rhizome, Poria Sclerotium, Moutan bark, Cinnamon bark, and aconite root.  . Statistically significant positive effect favoring intervention.
. Statistically significant positive effect favoring intervention. 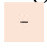 . Statistically significant negative effect favoring control.
. Statistically significant negative effect favoring control. . No statistically significant effect.
. No statistically significant effect.  . Statistical significance not tested. 5FU: Fluorouracil; C: chemotherapy cycle; CIPN: chemotherapy-induced peripheral neuropathy; CTX: chemotherapy; D: day; DEB-NTC: Neurotoxicity Criteria of Debiopharm; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-LC13: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Lung Cancer 13; EPA: eicosapentaenoic acid; FACT/GOG-NTX: Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity; GI: Gastrointestinal; hr: hour; min: minutes; mBPSPN: Modified Balis Pediatric Scale of Peripheral Neuropathies; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NSCL: non-small cell lung cancer; NR: not reported; UK: United Kingdom; USA: United States of America; wk: week; yrs: years.
. Statistical significance not tested. 5FU: Fluorouracil; C: chemotherapy cycle; CIPN: chemotherapy-induced peripheral neuropathy; CTX: chemotherapy; D: day; DEB-NTC: Neurotoxicity Criteria of Debiopharm; EORTC QLQ-C30: European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire; EORTC QLQ-LC13: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Lung Cancer 13; EPA: eicosapentaenoic acid; FACT/GOG-NTX: Functional Assessment of Cancer Therapy Gynecologic Oncology Group Neurotoxicity; GI: Gastrointestinal; hr: hour; min: minutes; mBPSPN: Modified Balis Pediatric Scale of Peripheral Neuropathies; NCI-CTCAE: National Cancer Institute Common Terminology Criteria for Adverse Events; NSCL: non-small cell lung cancer; NR: not reported; UK: United Kingdom; USA: United States of America; wk: week; yrs: years.
