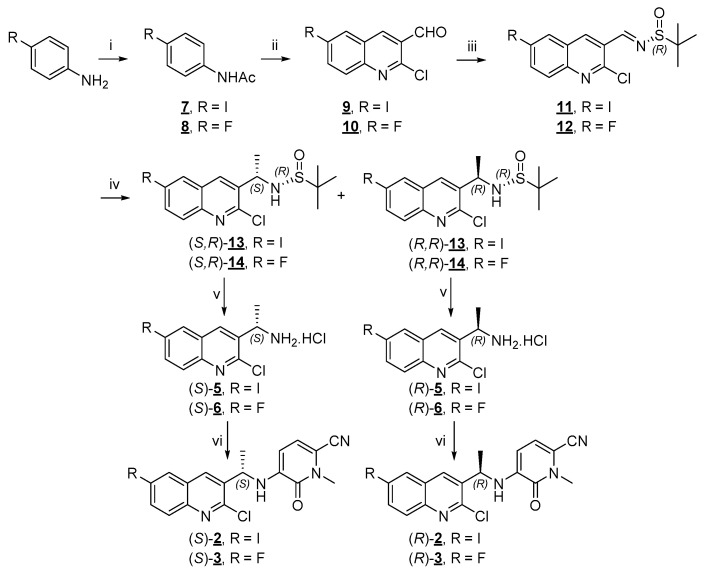
Scheme 2.
Synthesis of iodinated and fluorinated derivatives (S)-2, (S)-3 and their enantiomers. Reagents and conditions: (i) Ac2O, DIPEA, DCM, rt, 1–2.5 h; (ii) (a) POCl3, anh. DMF, 0 °C, then rt for 15–20 min; (b) 7 or 8, 80–85 °C, 16–19 h; (iii) ®-2-methylpropane-2-sulfinamide, DCE, CuSO4, 55 °C, 17 h—3 d; (iv) MeMgBr 3 M in Et2O, anh. DCM, −60 °C for 3 h, then −60 °C to rt overnight; (v) 1 N HCl aq., 1,4-dioxane, 105–110 °C, overnight; (vi) 4, DIPEA, DMSO, 110 °C, 15–22 h.