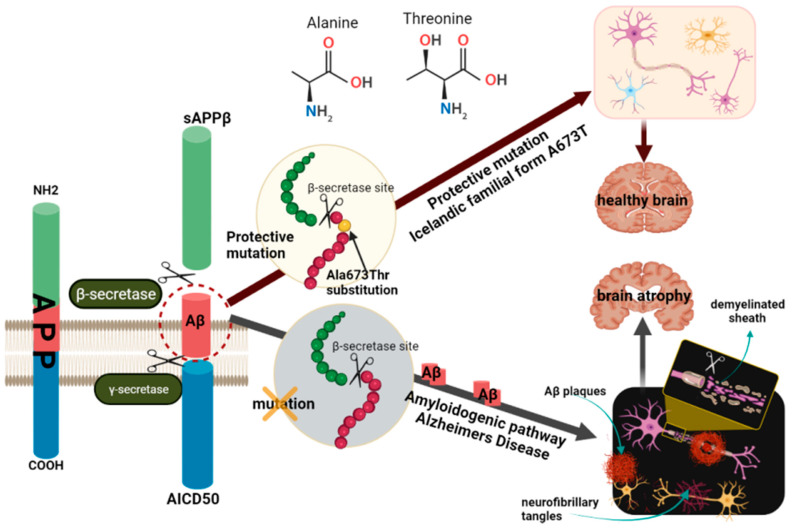
Figure 2.
Schematic representation of the molecular mechanisms of A673T protection against amyloid pathology. One of the pathways for APP processing is the formation of toxic amyloidogenic peptides, which accumulate and form amyloid plaques. Two cleavages are required for the release of Aβ from the APP molecule, one in the extracellular domain by β-secretase and another in the transmembrane region by γ-secretase. APP cleavage by β-secretase generates a fragment called β-APP and another smaller fragment that is embedded in the membrane until further cleavage in the presence of γ-secretase. The A673T protective mutation in the APP gene, located near β-secretase, encodes an alanine to threonine substitution. This mutation, also known as A2T, inhibits proteolytic cleavage at the cleavage site of APP by β-secretase. The absence of this mutation leads to cleavage in the presence of γ-secretase, leading to the release of Aβ peptides, especially Aβ42, and the formation of amyloid plaques. Neurofibrillary tangles also form, which together with β-amyloid plaques, lead to impaired synaptic transmission and ultimately to neuronal death. In the case of carriers of the A673T mutation, cleavage by γ-secretase with the formation of the Aβ42 peptide is thus avoided, the carriers being protected from developing AD. The figure was prepared with BioRender. AD, Alzheimer’s disease; APP, amyloid precursor protein; Aβ, amyloid-beta peptide.