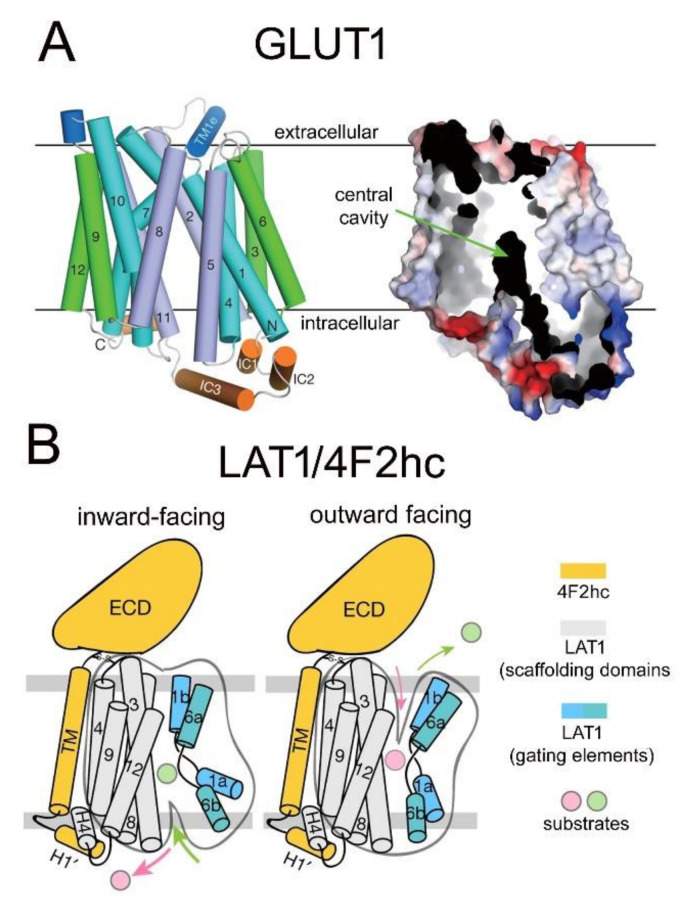
Figure 9.
Structure of GLUT1 and LAT1 carrier-mediated transporters. (A) (Left) Model of crystal structure of human GLUT1 showing orientation of 12 transmembrane regions (TMR) in four 3-helical repeat domains composed of TMRs 1,4,7,10 (blue), TMRs 3,6,9,12 (green), and TMRs 2,5,8,11 (purple); the extracellular and intracellular helices are shown in dark blue and orange, respectively. (Right) Surface electrostatic potential model shows a central transporter cavity. Reproduced with permission from [346], Copyright© 2014 Springer-Nature. (B) Inward-facing and outward-facing models of the LAT1-4F2hc heteroduplex. LAT1 is composed of 12 TMRs, which form scaffolding and gating domains. The 4F2hc is formed by an extracellular domain (ECD), a transmembrane (TM) domain, which binds to TMR4 of LAT1, and an intracellular loop (H1′). Reproduced with permission from [348], Copyright© 2019 Springer-Nature.