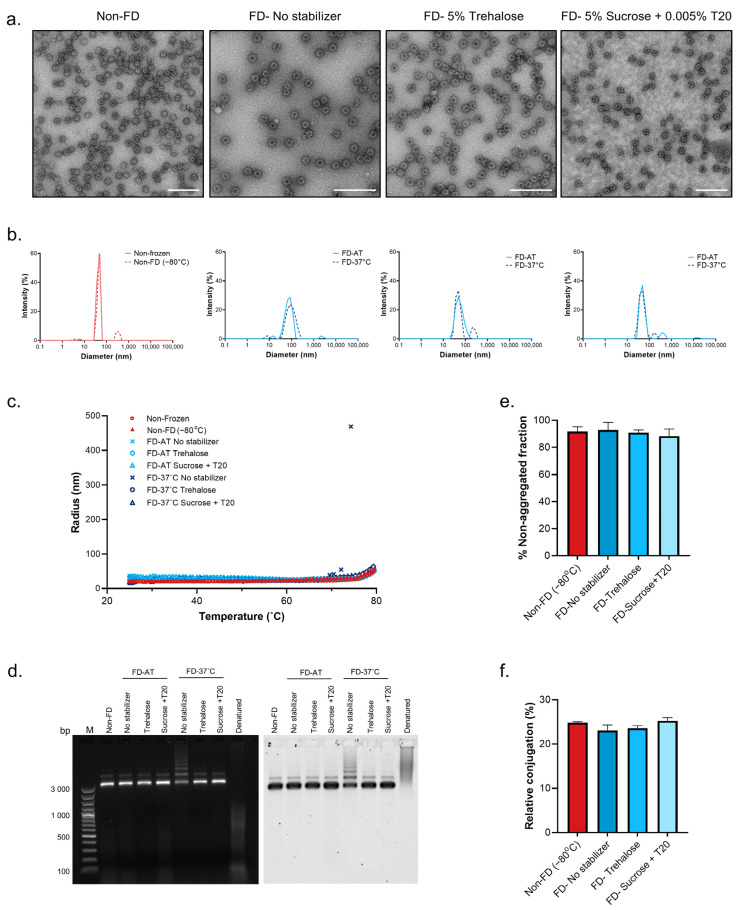
Figure 1.
Stability of freeze-dried and reconstituted Catcher-cVLPs. Catcher-cVLPs formulated in pure sodium phosphate buffer (pH 7.4) or with the addition of stabilizing excipients (5% trehalose or 5% sucrose + 0.005% Tween® 20 (T20)) were freeze-dried and subsequently stored at ambient temperature (FD-AT) or 37 °C (FD-37 °C) for 1 week (A) or 2 months (B–F). The stability of reconstituted freeze-dried material was compared to a non-frozen or a frozen non-freeze-dried (non-FD (−80 °C)) reference sample. (A) Negative stain transmission electron microscopy (TEM) images. Scale bar represents 200 nm. (B) Dynamic light scattering (DLS) analysis of reference samples (red) and freeze-dried Catcher-cVLP (blue) after storage at ambient temperature (solid line) or 37 °C (dashed line). (C) Thermal stability. The hydrodynamic radius of Catcher-cVLP was measured at increasing temperatures (from 25 °C and 80 °C) by DLS. (D) Agarose gel electrophoresis stained with ethidium bromide (left) and Coomassie brilliant blue (right). Native non-FD and FD samples were run in parallel to a denatured reference sample. (E) Quantification (by densitometric analysis of SDS-PAGE) of the relative amount (%) of reconstituted Catcher-cVLP, which remains in suspension after centrifugation (2 min at 16,000× G). (F) Quantification (by densitometric analysis of SDS-PAGE) of the relative conjugation of Catcher-cVLPs with a tagged antigen. Results show the mean and standard deviation of samples run in triplicate.