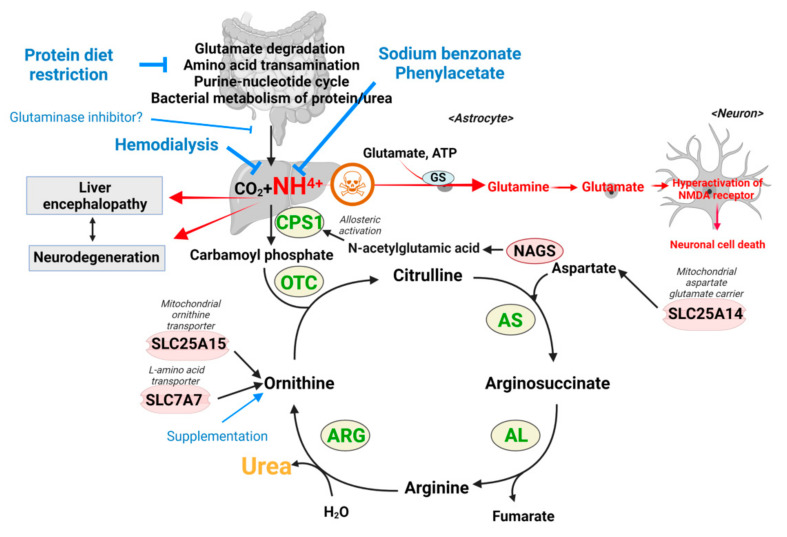
Figure 3.
Schematic diagram of urea cycle that detoxifies ammonia, toxicity mechanism of ammonia, and therapeutic options for hyperammonemia. Ammonia is produced throughout the cells of the body in numerous metabolic processes such as transamination reactions, glutamate degradation, and nucleotide metabolism. These systemic levels of ammonia are cleared by the urea cycle in the liver so that the nitrogen is ultimately excreted in the form of urea. Impairing mutations in the urea cycle enzymes leads to an inability to clear ammonia, leading to the disorder of hyperammonemia. Dysfunction of the liver, such as due to cirrhosis, can similarly lead to secondary hyperammonemia. In these contexts, the prevailing model is that ammonia is used to produce high levels of glutamine in astrocytes, causing their swelling, and also leading to glutamate formation in neurons to trigger excitotoxicity. Current strategies involve protein restriction to decrease ammonia formation, sodium benzonate and phenylacetate treatment to ‘chelate’ ammonia, or supplementation to activate the urea cycle. GS: glutamine synthase; CPS1: carbamoyl phosphate synthetase; OTC: ornithine transcarbamylase; AS: argininosuccinate synthetase; AL: argininosuccinic acid lyase AL; ARG: arginase; NAGS: N-acetylglutamate synthase; SLC25A15: mitochondrial ornithine transporter 1; SLC7A7: y + L amino acid transporter 1; SLC25A14: mitochondrial aspartate glutamate carrier 2.