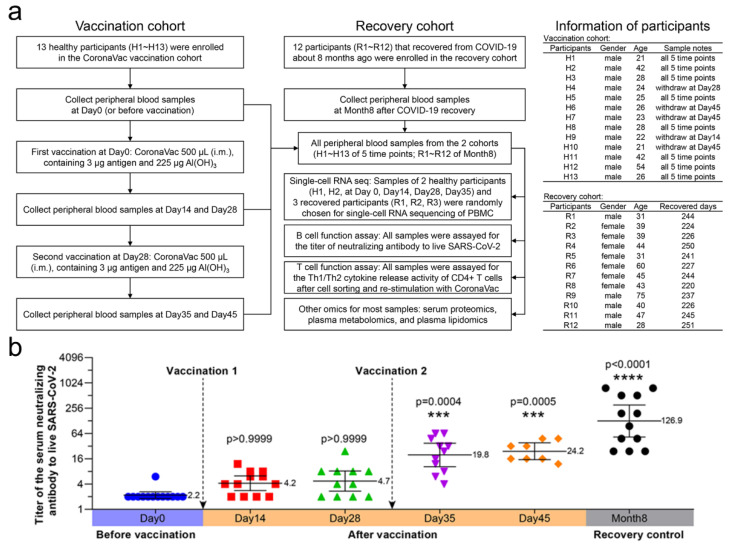
Figure 1.
Study profile and the neutralizing antibody titers of vaccinated and recovery participants. (a) Diagram showing the number of, and information regarding, participants in the study. Samples from 25 participants were assayed for serum-neutralizing antibody titers to live SARS-CoV-2 and cytokine release activity of CD4+ T cells. The participant information is shown on the right, including gender, age, withdrawing stage and recovered days. The samples from 25 participants were subjected to serum proteomics, plasma metabolomics, and lipidomics. After balancing the cost of scRNA-seq and the sample size of each group, participants H1 and H2 (each with four timepoints) and R1, R2, and R3 (each with one timepoint) were randomly chosen from each cohort, which made up 11 samples for scRNA-seq. (b) Titers of serum-neutralizing antibodies to live SARS-CoV-2 (n = 8–13 in each group). The solid lines represent a geometric mean with 95% confidence interval. Kruskal–Wallis test and Dunn’s multiple comparisons test were performed. Titers were compared with that of Day 0 or before vaccination. The geometric mean, p value, and p summary are annotated on the graph. *** p < 0.001; **** p < 0.0001.