Abstract
Non-alcoholic fatty liver disease (NAFLD) affects approximately 8 million Canadians. NAFLD refers to a disease spectrum ranging from bland steatosis to non-alcoholic steatohepatitis (NASH). Nearly 25% of patients with NAFLD develop NASH, which can progress to liver cirrhosis and related end-stage complications. Type 2 diabetes and obesity represent the main risk factors for the disease. The Canadian NASH Network is a national collaborative organization of health care professionals and researchers with a primary interest in enhancing understanding, care, education, and research around NAFLD, with a vision of best practices for this disease state. At the 1st International Workshop of the CanNASH network in April 2021, a joint event with the single topic conference of the Canadian Association for the Study of the Liver (CASL), clinicians, epidemiologists, basic scientists, and community members came together to share their work under the theme of NASH. This symposium also marked the initiation of collaborations between Canadian and other key opinion leaders in the field representative of international liver associations. The main objective is to develop a policy framework that outlines specific targets, suggested activities, and evidence-based best practices to guide provincial, territorial, and federal organizations in developing multidisciplinary models of care and strategies to address this epidemic.
Keywords: biomedical, Canadian NASH Network, clinical, diabetes, epidemiological, non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, public health
Introduction
Non-alcoholic fatty liver disease (NAFLD) is the most frequent liver disease at the global level, with an estimated prevalence of 25.24% (1). An estimated 7,800,000 Canadians are living with NAFLD (2). As the Canadian NASH Network (CanNASH), we modelled the burden of disease in Canada, demonstrating that NAFLD frequency will increase by 20% through 2030, when there will be an estimated 9,305,000 cases (2). There will also be an increase of 65% in cases of liver cirrhosis and hepatocellular carcinoma (HCC) related to NASH in the next decade (Figure 1). Despite these striking figures, Canada has no national strategy or models of care for addressing NAFLD.
Figure 1:
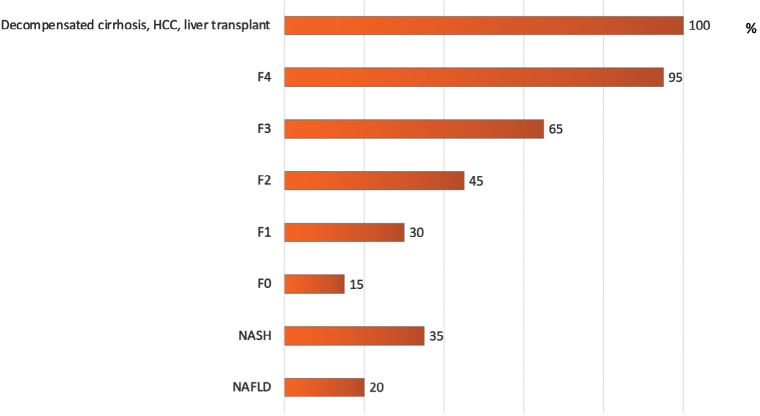
Percentage increase in prevalence of NAFLD, NASH, and associated liver fibrosis stages and complications in Canada from 2020 to 2030
HCC = Hepatocellular carcinoma; NASH = Non-alcoholic steatohepatitis; NAFLD = Non-alcoholic fatty liver disease
Data adapted from: Swain MG, Ramji A, Patel K, et al. Burden of nonalcoholic fatty liver disease in Canada, 2019–2030: a modelling study. CMAJ Open 2020;8:E429–36. https://doi.org/10.9778/cmajo.20190212. (2)
Moreover, a recent CanNASH survey showed that a significant proportion of Canadian physicians and nurses managing patients with liver disease are not very familiar with NAFLD, thus emphasizing the need for provider education, national practice guidelines, and improved treatment options (3) (Figure 2). This commentary summarizes highlights from the 1st International Workshop of the CanNASH, including insights into epidemiology and the association with metabolic syndrome and cardiovascular disease (CVD), pathophysiology and complications, diagnostics and biomarkers, therapeutic approaches, and case-finding in primary care settings. We also highlight research gaps and the need for a national strategy addressing this frequent and underdiagnosed disease.
Figure 2:
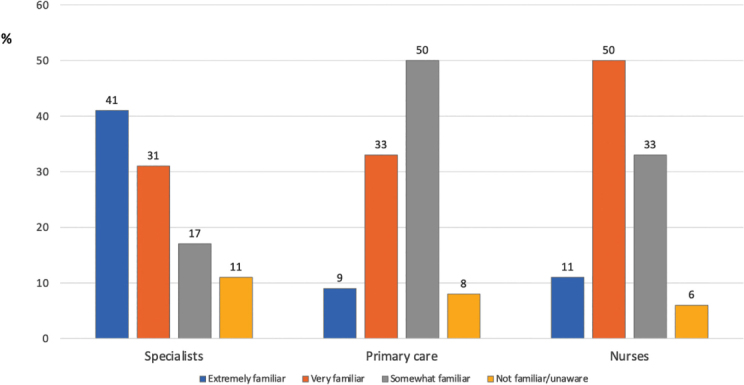
Level of awareness of NAFLD in Canada by specialty status
Data adapted from: Sebastiani G, Ramji A; Swain MG, Patel, K. A Canadian survey on knowledge of non-alcoholic fatty liver disease among physicians. Can Liv J. 2021;4:82–92. https://doi.org/10.3138/canlivj-2020-0033. (3) NAFLD = Non-alcoholic fatty liver disease
The Canadian NASH Network (CanNASH)
CanNASH is a national collaborative NASH research network seeking funding from public agencies and currently supported by industrial, private, and community organizations, as well as by non-governmental organizations (eg, Canadian Liver Foundation). It was established in October 2018 as a special interest group of the Canadian Association for the Study of the Liver (CASL) (https://cannash.ca). The network’s overarching goal is to build research capacity, translate evidence into practice policy, and promote a global effort against a silent killer poised to become one of the most important public health problems of the developed world by focusing on the themes of epidemiology, diagnostics, and prevention. The network brings together Canadian and international leaders in the field, nurses, trainees, community representatives, and practitioners at a multidisciplinary level with planning for progressive expansion.
The 1st International Workshop of CanNASH
This 2-day workshop—a joint conference between CanNASH and the single topic conference of CASL—was held virtually from Montréal, Québec on April 8–9, 2021. The overarching goal was to provide a multidisciplinary view of the current state of NASH research, treatment, and disease burden, both nationally and internationally, and establish the platform for collaborative research projects between Canada and institutional leaders in the field. The specific aims of the meeting were to:
provide an update on the latest advances in epidemiology, diagnostics, therapeutic and prevention strategies for NASH in Canada and internationally.
provide a forum for discussion and for the development of a roadmap for action to increase effective prevention, diagnostic, and treatment strategies for NASH in Canada.
identify research priorities and opportunities for scaling up the network and for collaborative grant applications.
disseminate workshop findings to support practice change, community awareness, harm reduction, and policy development.
facilitate transdisciplinary knowledge exchange and collaborations between Canadian trainees, established researchers, health care practitioners, policy makers, and community-based groups.
The presentations are available on the conference website, at https://event.fourwaves.com/cannash-casl-2021/pages.
Overview of the problem: Causes of lipotoxic liver injury and the burden of disease
NAFLD represents the most common chronic liver disease globally, with one in four adults affected (1). The increasing disease burden is driven by the epidemic of metabolic conditions, including type 2 diabetes (T2D) mellitus and obesity. The umbrella term NAFLD includes patients with both non-alcoholic fatty liver (NAFL) and NASH. NASH, defined as a specific pattern of hepatic steatosis and necroinflammatory histologic changes, affects about 20% of patients with NAFLD and can lead to progressive liver fibrosis, cirrhosis, and associated complications, namely liver failure and HCC (4) (Figure 3). NASH is the second leading indication for liver transplantation in North America, projected to become the first over the next 10 years (5), although alcoholic liver disease is also increasing as an indication for transplant (6,7). Dr Brent A Neuschwander-Tetri (Saint Louis University School of Medicine, St. Louis, Missouri, USA) opened the workshop by providing an overview of the burden of NAFLD, NASH, and its underlying pathogenesis. The development of progressive liver fibrosis is the most worrisome consequence of any chronic liver disease. One diagnostic goal is to identify NASH among those with NAFLD because having NASH increases the risk of progression to cirrhosis and helps identify treatment candidates. Because liver-related outcomes are much greater in patients with advanced fibrosis, a newer diagnostic goal is to identify patients with “at-risk” NAFLD, meaning those with F2–F4 fibrosis (8). Recent data from the Scientific Registry of Transplant Recipients in the United States showed that from 2002 to 2019, NASH was the most rapidly increasing indication for liver transplantation in patients without and with HCC (7).
Figure 3:
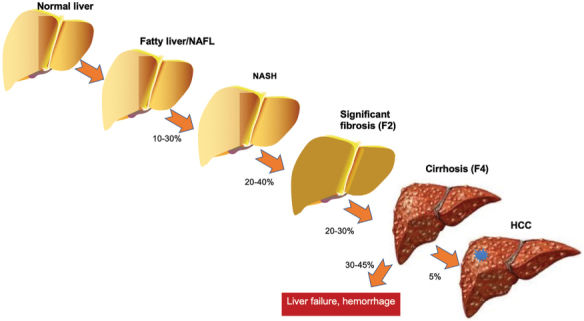
Pathogenesis and progression of NASH and associated liver fibrosis
NASH = Non-alcoholic steatohepatitis; NAFL = Non-alcoholic fatty liver; HCC = Hepatocellular carcinoma
NAFLD without cirrhosis also contributes to the increase in NASH-related HCC (7). Markov modelling of disease burden, based on increases in T2D and obesity, predicts marked increases in decompensation, deaths, and HCC related to NASH by 2030 in the United States (9). There is a very close relationship between NAFLD and insulin resistance (IR), and NAFLD likely occurs due to IR rather than being its cause. This is supported by the fact that polymorphisms in APOB and PNPLA3 genes associated with hepatic steatosis are not associated with IR (10–12). The primary underlying cause of IR in the body appears to be adipose tissue that is stressed by a supply of nutrients that exceeds its storage capacity (13,14). This may explain why lean NAFLD can occur in the setting of poor adipose tissue storage capacity, such as the lipodystrophies, leading to metabolic abnormalities despite normal BMI. NASH can be viewed as lipotoxic liver injury in which free fatty acids (FFAs) in the liver, predominantly derived from insulin-resistant adipose tissue, and to a lesser extent from glucose and fructose in the diet, with an even smaller contribution of dietary fat intake to liver FFAs, contribute to the phenotype of NASH. When FFAs are delivered to the liver in excess, they can be metabolized to lipotoxic lipids, inducing hepatocyte endoplasmic reticulum stress and apoptosis, resulting in inflammation and fibrosis in the liver (11,15–19). These pathways can be further modified by multiple other factors, such as genetic polymorphisms in metabolic and inflammatory pathways, the gut microbiome, cholesterol levels, and the periodic hypoxia of sleep apnea. This complex pathogenesis of NASH helps identify multiple possible sites for therapeutic intervention in drug development (20).
Pathophysiology and complications
The pathogenesis of NASH is a complex intersection between IR, oxidative stress, genetics, and environmental factors. This multifactorial process leads to both liver-related complications, mostly associated with the development of cirrhosis and portal hypertension, and to extrahepatic diseases such as CVD and non-liver cancer (4). Prof Massimo Pinzani (Institute for Liver and Digestive Health, University College London, London, UK) opened the session by reviewing the mechanisms of fibrogenesis in NAFLD while highlighting that there may be two parallel disease progressions. Of the individuals with NAFLD, 20% will develop NASH, and a further 20% of NASH patients will progress to cirrhosis (4). However, a smaller proportion of patients with NAFL (4%) can progress to cirrhosis without showing definite histological evidence of NASH (ie, presenting only steatosis and fibrosis with no signs of tissue inflammation); this suggests a distinct and parallel disease progression that cannot be diagnosed as NASH but is still important for clinical outcomes. The mechanisms of fibrogenesis in NAFLD include oxidative stress, altered intestinal permeability, genetic factors, and the complex hepatic inflammatory network. In NASH, reactive oxygen species derived from various sources, such as hepatocyte injury, can lead to lipid peroxidation and the formation of reactive aldehydes. Reactive aldehydes can directly induce collagen synthesis in human hepatic stellate cells (HSC), suggesting a direct mechanism through which reactive oxygen species promote fibrogenesis (21). Moreover, increased intestinal permeability in obesity allows for increased entry of pathogen-associated molecular patterns (eg, lipopolysaccharides) into the portal circulation, which, upon arrival at the liver, can activate toll-like receptor 4 on HSCs to induce pro-fibrogenic and pro-inflammatory effects (22). An increased risk of NASH, severe fibrosis, and HCC is associated with the PNPLA3 variant rs738407 C> G (I148M) (23). PNPLA3 expression is required for HSC activation, and the variant PNPLA3 I148M confers a pro-inflammatory and pro-fibrogenic phenotype in human HSCs (24). Data from Prof Pinzani’s team show that primary human HSCs homozygous for the PNPLA3 I148M variant have a dysregulated oxidative stress response compared to HSCs heterozygous for the variant or homozygous for the wild-type allele. A network of immune cell types contributes to HSC activation and liver fibrogenesis, and cenicriviroc (anti-CCR2/CCR5) is an example of a strategy in which the NASH inflammatory network has been targeted. Overall, considering the complexity of the fibrogenetic process in NASH, the ideal target for treatment should likely be a molecule involved in metabolism, inflammation, and liver fibrosis.
NAFLD has been long regarded as a hepatic manifestation of metabolic syndrome. However, accumulating evidence indicates that the effects of NAFLD extend beyond the liver and are negatively associated with a range of chronic diseases, most notably CVD, T2D, and cancer. These diseases result from the same underlying pathophysiological processes associated with metabolic syndrome, such as IR, chronic systemic inflammation, and dyslipidemia (Figure 4). Dr Salvatore Petta (University of Palermo, Palermo, Italy) provided an overview of NASH as a multi-organ disease. A recently proposed new nomenclature for the disease would move from NAFLD, based on “negative criteria,” to metabolic associated fatty liver disease (MAFLD), based instead on “positive criteria.” This proposed definition of MAFLD is based on the presence of hepatic steatosis in addition to one of the three following conditions: overweight/obesity, T2D, or metabolic dysregulation (25). This new definition would also help emphasize NASH as a multi-organ disease and its association with extrahepatic conditions. CVD represents the most frequent cause of death in NAFLD patients, followed by non-hepatic cancer and complications from end-stage liver disease (cirrhosis and HCC) (26).
Figure 4:
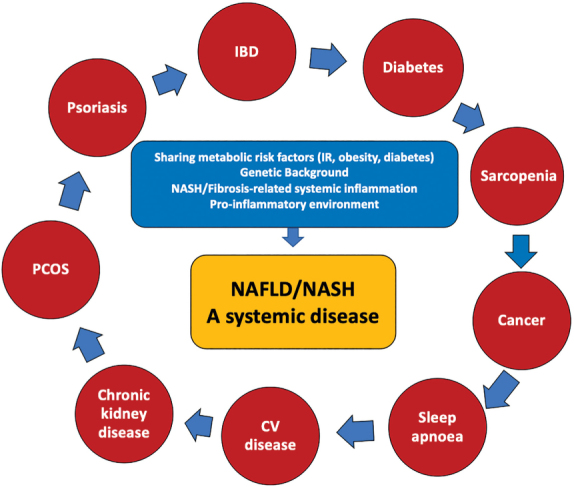
Association between NAFLD and extrahepatic manifestations
NAFLD = Non-alcoholic fatty liver disease; NASH = Non-alcoholic steatohepatitis; CV = Cardiovascular; IBD = Inflammatory bowel disease; IR = Insulin resistance; PCOS = Polycystic ovary syndrome
The rate of extrahepatic deaths related to CVD, T2D, and cancer in NAFLD patients increased between 2007 and 2017 (27). Moreover, compared to the general population, NAFLD patients have the highest relative risk of dying from HCC, followed by cirrhosis and CVD (28). Patients with NAFLD have a 2.2 higher risk of developing T2D than those without (29). In patients with T2D, the presence and severity of NAFLD increase the risk of early diastolic dysfunction (30). Hepatic steatosis is associated with a higher prevalence of carotid plaques, with a reported odds ratio of 1.63 (31). A genetic contribution to the relationship between NAFLD and CVD has been suggested. TM6SF2 single-nucleotide polymorphism (E167K) associates with increased NASH but a lower prevalence of carotid plaques (32). Patients with this single-nucleotide polymorphism are at increased risk of advanced fibrosis but a reduced risk for carotid plaques. TM6SF2 encodes a protein involved in lipid export from the liver.
NAFLD patients have a 1.6 times higher risk of developing incident cardiovascular events compared to those without NAFLD (33). NAFLD also increases the risk of HCC and non-hepatic cancers, such as colorectal and breast cancer (34). The association between NAFLD and metabolic comorbidities is of a bidirectional kind. Indeed, in 1,051 participants of the Framingham heart study, NAFLD increased the risk of developing T2D and hypertension, and T2D and hypertension, in turn, increased the risk of developing NAFLD (35). NAFLD severity also plays a major role in the risk of metabolic comorbidities. The incidence of T2D increases with increasing stages of liver fibrosis in NAFLD (36). The presence of significant liver fibrosis also increases the risk of developing arterial hypertension (37). The prevalence of carotid atherosclerosis and left ventricular alterations is increased with higher liver fibrosis stages (38,39). The risk of cardiovascular events is two times higher in patients with severe NAFLD compared to non-severe (33). A competitive risks approach should be used to evaluate the risk of liver-related events and extrahepatic events together, as opposed to separately, which is currently being done. Unpublished data from Dr Petta’s team suggest that in patients with mild fibrosis (F0–F1), the risk of hepatic events is low while that of extrahepatic events is high. In patients with significant fibrosis (F2), the risk of hepatic events remains low but clinically relevant while that of extrahepatic events is high. In patients with advanced liver fibrosis (F3–F4), the risk of both hepatic and extrahepatic events is high.
Interestingly, patients with fibrosis stage F3 are at higher risk of vascular events and extrahepatic cancers than liver-related events, while patients with fibrosis stage F4 (cirrhosis) are at higher risk of liver-related events than vascular events and extrahepatic cancers (40). Overall, NAFLD is highly prevalent in the general population, which parallels the epidemic of obesity and T2D. Importantly, NAFLD patients are at high risk of both liver-related and extrahepatic events and death. A multifactorial mechanism of metabolic comorbidities, genetic background, and severity of liver disease can modulate the risk of extrahepatic complications in NAFLD. The impact of investigational drugs for NASH should consider both hepatic and extrahepatic outcomes.
The end-stage liver complications occurring in NASH are those associated with sequelae of portal hypertension and HCC, two conditions driving most of the prognosis in NASH. Dr Annalisa Berzigotti (University of Bern, Bern, Switzerland) showed that the risk of decompensation in NASH cirrhosis is lower compared to that of hepatitis C virus (HCV)-related cirrhosis, but once decompensation occurs, survival is the same for both etiologies (41). Cirrhotic patients (stage F4) have increased hepatic venous pressure gradient (HVPG). The presence of clinically significant portal hypertension (defined as HVPG ≥10 mmHg) predicts clinical events and progression to decompensation, HCC risk, and development of varices and ascites (42). Some liver function tests (albumin), MELD score, and obesity can also independently predict this progression (43,44). Interestingly, HVPG values in NASH patients are lower than those in HCV patients when matched by fibrosis stage (45). In NASH, there is an increased risk of liver-related events per 1 mmHg increase in HVPG over time (42). While there is good agreement between wedged hepatic venous pressure and portal pressure in alcoholic and HCV-related cirrhosis, one-third of patients with NASH-related cirrhosis show a disagreement between wedged hepatic venous pressure and portal pressure (mostly underestimation). In NASH, there may be a presinusoidal component to portal hypertension, at least in some cases, that is not captured by HVPG (46). This was important in the simtuzumab studies, a monoclonal antibody directed against lysyl oxidase-like 2, as 14% of patients with HVPG ≤10 mmHg developed portal hypertension-related complications, which did not occur in the timolol study (for patients with alcoholic and HCV-related liver disease) (42). Non-invasive tests can be used to predict the risk of liver-related events. For example, a liver stiffness measurement (LSM) of >21 kPa by transient elastography (TE) using FibroScan (Echosens, Paris, France) combined with the fibrosis biomarker Fibrosis-4 (FIB-4) >2.67 can be used to identify patients at risk for liver-related events (47). The Baveno criteria and the slightly more expanded NAFLD cirrhosis criteria, based on platelet count and LSM, can rule out varices needing treatment while missing less than 5% of varices that do need treatment (48). Portal vein thrombosis occurs more frequently in NASH compared to HCV-related cirrhosis (49). Moreover, renal dysfunction is usually more severe in NASH cirrhosis and requires more often renal replacement therapy, while NASH cirrhosis is an independent risk factor for renal replacement therapy after transjugular intrahepatic portosystemic shunt (50,51). Sarcopenia and myosteatosis are common in patients with NASH cirrhosis and are independently associated with higher mortality (52). As for HCC, the incidence in patients with NASH cirrhosis is lower than other etiologies of liver disease, such as chronic hepatitis B (53). However, because the burden of NAFLD is much higher than chronic hepatitis B, NASH is the major indication for HCC requiring liver transplant (54). HCC can develop in both cirrhotic NASH (incidence 0.3%–2.6% per year) and in non-cirrhotic NAFLD/NASH (unclear incidence, but estimates 0.04%–0.3% per year) (55). HCC in NAFLD occurs more often in the absence of cirrhosis compared to other etiologies of liver disease (34.6% of HCC cases are on a non-cirrhotic background, while in HCV, HBV, and alcohol ≥88% occurs on a cirrhotic background) (56). Moreover, patients with NAFLD-related HCC are less likely to undergo HCC surveillance in the 3 years leading up to the diagnosis compared to those with alcohol or HCV-related HCC (56,57). Ultimately, this results in a delay in diagnosing HCC as compared to HCV: 54% of patients with NAFLD-related HCC are diagnosed at a stage beyond a curative option, compared to 44% of patients with HCV-related HCC (57). In patients with liver cirrhosis, the main factors associated with increased risk of HCC are male sex, obesity, and T2D (58). In the specific setting of NAFLD-related liver cirrhosis, an increased risk of HCC has been observed with older age, presence of T2D, and low albumin (59).
Diagnostics and biomarkers
Liver biopsy is considered the gold standard of reference for diagnosing NASH and associated liver fibrosis (60). Despite many research efforts to identify an accurate serum biomarker, a diagnosis of NASH is still based on the histologic coexistence of hepatic steatosis, necroinflammatory changes, and hepatocyte ballooning. However, liver biopsy is invasive, costly, and prone to sampling error (61,62). Although histology is still necessary for a definitive diagnosis of NASH, the coexistence of hepatic steatosis and liver fibrosis may indicate the presence of NASH. Several non-invasive tools for the diagnosis of NAFLD and associated liver fibrosis have been extensively studied. These methods rely on two different approaches: a biological approach based on the quantification of biomarkers in the serum, and a physical approach based on LSM by either ultrasonographic elastography techniques or magnetic resonance elastography (MRE) (Figure 5) (63). Prof Elizabeth M Brunt (Washington School of Medicine, St. Louis, Missouri, USA) provided a detailed overview of the role of liver biopsy and histology in NAFLD. NASH is a driver of liver fibrosis, but whether NAFL as steatosis itself can progress to fibrosis directly remains unknown. In 2005, the NASH CRN pathology committee published the scoring system that is now well-known (64). The lesions for activity are known as grade and are referred to as the NAFLD activity score (NAS). The score ranges from 0 to 8 and is comprised of steatosis (0–3), lobular inflammation (0–3), and ballooning (0–2). Stage refers to fibrosis, but it is not just matrix; it also includes the location of fibrosis as well as parenchymal remodelling. Bridging fibrosis and cirrhosis are the most important stages and are therefore referred to as advanced fibrosis, associated with the risk of end-stage liver complications and HCC. The diagnostic categories that are used by pathologists for NASH include the following: not NAFLD, NAFL, definite steatohepatitis, borderline steatohepatitis with either zone 1 or zone 3 accentuation, and cryptogenic cirrhosis. Although tarnished, liver biopsy is still the gold standard for diagnosing NASH, as its diagnosis is more than a simple addition of lesions. In a histologic study of 934 non-cirrhotic adults, the NASH CRN demonstrated that 25% of definite steatohepatitis diagnosed by the pathologist had an NAS score <4, which would have been missed by the simple use of the NAS score (65). Interestingly, while both an NAS score ≥5 and a steatohepatitis diagnosis were associated with alanine aminotransferase (ALT), aspartate aminotransferase (AST), and homeostatic model assessment (HOMA)-IR on regression analysis, only steatohepatitis (but not an NAS score ≥5) was significantly associated with diabetes and metabolic syndrome. These results indicate that pathologists see something in NASH that is important and not represented only by NAS. As with other scoring systems in chronic liver diseases, NAS was created to evaluate lesions of significance for semi-quantitative assessment and assign relative, graduated values to represent severity. None of these scores were developed to replace diagnosis. Pathology diagnoses are not made by simple addition, although existing lesions are important parts of the composite. As such, NAS and steatohepatitis diagnosis serve two important purposes—they are related but not interchangeable. Scoring is indeed different from diagnosis. The descriptors include steatosis, lobular inflammation, ballooning, and fibrosis; however, the diagnosis also includes the pattern of injury. The initial injury pattern usually occurs in zone 3. There are several patterns of liver fibrosis in the adult liver: stage 1 (zone 3 perisinusoidal fibrosis), with stage 1a versus 1b depending on the density of the fibres in zone 3; stage 2 (combination of zone 3 perisinusoidal fibrosis and portal/periportal fibrosis); stage 3 (bridging fibrosis between any vascular structures); and stage 4 (cirrhosis, which may or may not retain features of active steatohepatitis). Another important concept is that not all clinical NASH turns out to be NASH on liver biopsy. A study of 354 patients with unexplained liver test abnormalities found that 66% had fatty liver (of which 32% had steatohepatitis with liver fibrosis), but about 13% could be explained by other liver diseases, 9% were cryptogenic, and nearly 6% had normal liver histology (66). Biopsy is also helpful when NASH occurs concurrently with other forms of chronic liver disease, such as chronic hepatitis B and C, hepatotoxicity caused by drugs or occupational toxins, and primary biliary cholangitis. A major diagnostic complication is the alcohol conundrum, which occurs when there are risk factors for NASH, namely obesity and T2D, and significant alcohol intake. Histologically, macro, mixed, and large droplet steatosis occur in both alcoholic liver disease and NAFLD. However, sclerosing hyaline necrosis, venocclusive-like lesions, acute cholestasis in the non-cirrhotic liver, and alcoholic foamy degeneration are lesions occurring only in alcoholic liver disease (67). Liver biopsy has also been helpful to show that the spectrum of NAFLD is two-way, as it can show the progression of NAFL/NASH, documented in 30% of cases, as well as regression, documented in 20% of cases (68,69). Regression of NAFL/NASH has been shown in clinical trials, especially in the placebo arms, and the strongest correlate for fibrosis improvement was NASH improvement (69). Liver biopsy evaluation is also very important in treatment trials as it is a surrogate for hard outcomes, namely cirrhosis, HCC, liver transplant, and death. Both the US Food and Drug Administration (FDA) and the EU Medicines Agency (EMA) require histologic efficacy for late phase II and phase III registration trials, but specific lesion requirements have proven challenging. These lesions were based on initial evaluations of glass slides, but now digitized images are used in trials. Poor pathologist agreement may affect enrolment and evaluation of efficacy in clinical trials (70). The insulin sensitizer MSDC-0602K did not demonstrate a significant effect on primary and secondary liver histology end points in a phase IIb NASH trial; however, 46% of re-read entry biopsies did not meet entry criteria (70,71). Prof Brunt commented on the importance of involving pathologists from the beginning of the trial and having consensus reads (72). Another possible approach may be to assess for improvement instead of insisting on calculating an algorithmically determined resolution. Artificial intelligence (AI) and machine learning approaches have been proposed (73,74). The value of AI in NASH may lie beyond replicating NAS and fibrosis stage as a guide for less experienced pathologists/hepatologists for lesion location and help with the actual diagnosis. It may also be helpful for quantitative analysis of features, particularly the intersection of stages 3–4 and pre-/post-treatment comparisons, and for deeper analysis of qualitative features, such as ballooning. Overall, AI allows to see things not available to the human eye and to improve reproducibility. On the positive side, non-invasive tests continue to improve.
Figure 5:
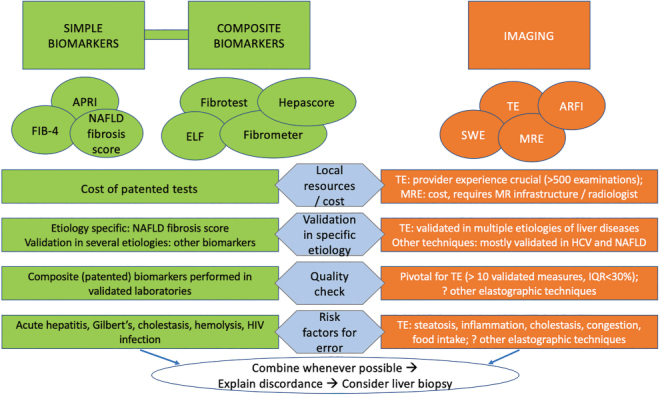
Use of non-invasive tests for the diagnosis of liver fibrosis in clinical practice
APRI = AST-to-platelet ratio index; FIB-4 = Fibrosis-4 score; NAFLD = Non-alcoholic fatty liver disease; ELF = Enhanced liver fibrosis score; SWE = Shear-wave elastography; TE = Transient elastography; ARFI = Acoustic radiation force impulse; MRE = Magnetic resonance elastography; HCV = Hepatitis C virus; IQR = Interquartile range
Dr Keyur Patel (University Health Network, Toronto, Ontario, Canada) provided an update on serum biomarkers to diagnose NASH and advanced fibrosis. Among these, several blood-based biomarkers have been evaluated for the differentiation of NASH from simple steatosis, including various acute-phase proteins and cytokines, along with markers of apoptosis, metabolic signalling, and oxidative stress. A systematic review of 122 studies that included 219 different blood markers (107 single markers, 112 scoring systems) to differentiate simple steatosis from NASH concluded that no single test or combinations of tests met the minimum benchmark for a diagnostic test with pooled sensitivity or specificity of ≥80% (75). Measuring keratin 18 fragments (CK-18), either as the cleaved fragment M30 or intact protein M65, as a marker of hepatocyte apoptosis has been proposed as a marker for diagnosis of NASH. Pooled data from 15 studies for M30, and 5 studies for M65, indicated sensitivity and specificity for NASH diagnosis of 68% and 74% for M30, and 76% and 74% for M65, respectively (75). At the current time, no blood marker can be recommended for differentiating simple steatosis from NASH. Newer combinations of markers for diagnosis of fibrotic NASH (NAS ≥4 and F ≥2) have been proposed but require further validation and are not yet readily available. These include FibroScan–AST (FAST) score (LSM by TE, controlled attenuation parameter [CAP], AST) (76), MACK 3 (AST, HOMA-IR, CK-18 M30) (77), and NIS4® (GENFIT SA, Nord, France) (miR-34a-5p, YKL-40, alpha-2-macroglobulin, hemoglobin glycosylated) (78). Several patented and non-patented combined serum biomarker algorithms have been developed to predict advanced fibrosis in NAFLD patients. The NAFLD Fibrosis Score and FIB-4 are the most validated of the non-proprietary tests due to the use of simple, readily available tests and free online calculators. The AASLD Guidance statement on NAFLD stated that NAFLD Fibrosis Score or FIB-4 are clinically useful tools for identifying NAFLD patients with a higher likelihood of advanced fibrosis (4). However, these tests are associated with “indeterminate” range scores with poor diagnostic performance in one-third of cases. Patented marker panels such as Enhanced Liver Fibrosis (ELF) (Siemens Healthineers, Erlangen, Germany) are available in Europe for screening NAFLD patients and recommended by the UK’s National Institute for Health and Care Excellence (NICE) guidance for diagnosing advanced fibrosis in patients incidentally identified with NAFLD (79). However, the thresholds for F3–4 recommended by NICE (ELF ≥10.51) differ from the assay manufacturer (ELF >9.8). A recent meta-analysis of 11 studies that evaluated ELF indicated a high sensitivity, but limited specificity for excluding F3-4 at ELF <7.7. The diagnostic performance of ELF at higher thresholds was limited in low prevalence settings (positive predictive value of 0.26 at ELF ≥10.51 for F3–4 prevalence of 5%) (80). Data from phase III NASH clinical trials have further highlighted the limitations of serum markers as single tests for advanced fibrosis. In the screening cohort from the phase III study, selonsertib (inhibitor of apoptosis signal-regulating kinase 1), with >70% prevalence of F3–4, ELF was associated with indeterminate rates of 45%, with an additional misclassification rate of 19% (81). In the phase III REGENERATE study (Randomized Global Phase 3 Study to Evaluate the Impact on NASH With Fibrosis of Obeticholic Acid, OCA, a farnesoid X receptor agonist, FXR), screening cohort data with ELF available in n = 1,170 patients at F3–4 prevalence of 43%, also noted high indeterminate rates of 59% (82). Given these diagnostic limitations of single markers, sequential algorithms are being applied to indeterminate results from simple tests such as FIB-4 to improve test specificity in low prevalence cohorts. In a Canadian study of 541 biopsy-proven NASH patients from two tertiary centre cohorts, sequential FIB-4 followed by NAFLD fibrosis score significantly reduced indeterminates and avoided biopsy for F3–4 in 27%–29% of patients (83). In a low prevalence cohort, sequential application of FIB-4 and ELF in a UK primary care NAFLD population reduced referrals to specialist care by 80% (84). Currently available serum markers for fibrosis assessment were developed in cross-sectional cohorts and appear to have limited diagnostic utility in following changes in fibrosis. A systematic review of 6 studies with variable time to follow-up in assessing NAFLD fibrosis progression or regression indicated AST-to-platelet ratio index (APRI), FIB-4, and NAFLD fibrosis scores had inconsistent performance for predicting change in the fibrosis stage (85). Data from a phase II study of simtuzumab that enrolled stage F3–4 patients but failed to achieve histologic end points of treatment efficacy has provided useful information on the association of serum markers with the natural history of disease progression or regression. In this study, higher ELF scores in F3 patients at baseline were associated with progression to cirrhosis at week 96. Conversely, lower baseline ELF, FIB-4, NFS, and APRI scores in F4 patients, but no change in index scores, were associated with cirrhosis regression at the week 96 biopsy follow-up (42). Pooled data from the simtuzumab and selonsertib trials that enrolled F4 patients have indicated that cirrhosis regression is associated with reduced liver-related events, and baseline ELF, FIB-4, and NAFLD fibrosis score, were associated with risk of clinical events during median follow-up of 16 months (86). These results require further validation to define optimal serum test thresholds predictive of histologic change or clinical outcomes in real-world cohorts. Emerging future diagnostic biomarker tools for NASH and fibrosis include the highly multiplexed SOMAScan proteomic assay (SomaLogic Inc., Boulder, Colorado, USA). These newer technologies appear promising to develop the next generation of blood biomarkers as part of a liquid biopsy, but still require further validation.
Ultrasound-based generation of shear waves can be used to non-invasively measure liver stiffness, as outlined by Dr Mark Swain (University of Calgary, Calgary, Alberta, Canada). Increasing liver stiffness, in general, correlates with increasing liver fibrosis severity. Elastography-based approaches are being increasingly used to direct many aspects of NAFLD patient care. In the context of NAFLD clinical care pathways, especially within primary care, elastography can be used for patient case identification strategies to better find those NAFLD patients with an increased likelihood of having more advanced liver disease (ie, >F2) and who may potentially benefit from specialist referral/care. Within this patient care paradigm, those NAFLD patients who are found to have lower LSM and therefore lower risk for having significant liver fibrosis (ie, with low risk of progressive disease; <F2), could be cared for within their primary care home (and not referred to a liver specialist). This NAFLD patient group could be re-tested, at designated intervals (~3 years), to ensure a lack of liver disease progression. In addition, elastography techniques can be used by specialists as a therapeutic decision-making tool. They will likely become a mainstay for patient-related decisions regarding starting (or stopping) directed NAFLD therapies when they become clinically available in the next few years. Three main elastography techniques are currently clinically available. TE has been widely available for clinical use for some years (more recently in the United States). This technique uses a freestanding dedicated machine to generate shear waves, measuring the propagation of low frequency (50 Hz) shear waves through the liver to define liver stiffness: the faster the shear-wave propagation, the stiffer the liver. Importantly, for larger patients with a skin-to-liver capsule distance of >25 mm, an XL probe is required to obtain accurate results using the FibroScan. Point shear-wave elastography (such as acoustic radiation force impulse) is another technology with the advantage that it can be integrated into most commercially available (ie, community-based) ultrasound machines by software upgrade. This technique measures liver stiffness by applying a single acoustic pulse after identification of a region of interest within the liver using B-mode visualization. 2D shear-wave elastography technology can also be readily added to commercially available ultrasound machines. This technique generates multiple shear waves, and their propagation through the liver can be visualized and measured in real time. Importantly, all the elastographic techniques sample a relatively small area of liver tissue for measuring liver stiffness (<2 cm). Optimal liver fibrosis threshold cut-offs are being better defined (87); however, the technique failure rate is known to increase with increasing BMI (especially >40 kg/m2), and this will become progressively problematic as the world population gets larger with each passing year. Primary care-directed 2D shear-wave elastography-based NAFLD case-finding pathway was implemented in Calgary, Alberta, Canada, in 2016 (88). This pathway employed a 2D shear-wave result of <8 kPa as a decision point to define NAFLD patients at low risk for significant liver fibrosis and who could be managed within their primary care home. Importantly, the use of this pathway showed that >90% of NAFLD patients had a shear-wave value of <8 kPa and did not require specialist referral. Moreover, in this pathway, a BMI of >37 kg/m2 increased the likelihood of a shear-wave value of >8 kPa, as did the presence of hypertension and a hemoglobin glycosylated result of >6.2%. Interestingly, the presence of abnormal serum transaminases (ALT, AST) within 2 years prior to the shear-wave assessment was not linked to a finding of an elevated shear-wave result of >8 kPa. CAP is an additional variable that can be generated by the FibroScan technology, and it allows for the quantification of liver fat content (89). To do this, the machine generates an acoustic impulse that passes through liver tissue. Fat within the liver attenuates the acoustic impulse signal, and the more fat present, the higher the attenuation. The degree of attenuation is reported as a CAP score that varies from normal (~220) to a maximum score of 400 (severe steatosis). In summary, elastography-based techniques are increasingly being employed as tools for case-finding of NAFLD patients with significant liver fibrosis in the general population and for clinical decision-making processes within liver specialty care, including the decision to undertake a liver biopsy and/or to define patient treatment strategies.
While liver biopsy is the definitive test for NASH diagnosis and for the staging of liver fibrosis, it is neither practical nor feasible to perform for NAFLD considering that it affects 20%–30% of the adult North American population. Imaging offers the possibility to sample the liver using different modalities. While dual-energy computed tomography (CT) has been studied for fat quantification and imaging of liver fibrosis, ultrasound and magnetic resonance imaging (MRI) are more commonly used for assessment of chronic liver disease, especially for assessment of liver fibrosis. Dr An Tang (Université de Montréal, Montréal, Québec, Canada) focused on the role of MRI for assessment of the NAFLD spectrum. Histopathological changes are accompanied by changes in physical properties of the liver that can be measured to quantitate liver fat, inflammation, and fibrosis. Unlike biopsy sampling, MRI can cover the entire liver and account for disease heterogeneity. The interest in using MRI to assess the NAFLD spectrum stems from the potential for a comprehensive diagnostic test to quantitate each hallmark feature of NAFLD; it does so by leveraging different physical concepts and including a larger area than possible with liver biopsy. MRI can be used to assess liver fat for four purposes: detection, grading, assessment of distribution, and determining the composition of triglycerides. While MR spectroscopy was the non-invasive reference standard and considered the most accurate technique for fat quantification due to its spectral resolution, it has limited availability, is time-consuming, and requires spectroscopy expertise. Over time, MRI proton density fat fraction (PDFF) has become more popular because it is in close agreement with MR spectroscopy (minimal bias: –0.13% and 95% limits of agreement: ± 4%), rapid to perform, and covers the entire liver (90). However, it currently does not permit the assessment of the type of triglycerides. MRI-PDFF provides good classification accuracy for grading liver steatosis compared to histopathology (91). Interestingly, patients with NAFLD have liver triglycerides that become more saturated as PDFF increases (92). In other words, patients with a lot of liver fat also have bad fat. In general, elastography techniques cannot measure stiffness directly; rather, they assess stiffness indirectly by measuring the speed of shear waves propagating in the tissue of interest. The underlying concept is that shear-wave speed is related to tissue stiffness: shear waves travel slowly in soft tissues and faster in stiff tissues. MR elastography (MRE) requires phase-contrast pulse sequences synchronized with a mechanical driver system to generate wave images and elastograms (ie, stiffness maps) of the liver (93). A recent Japanese study performed a head-to-head comparison of MRE, shear-wave elastography, and TE in patients with NAFLD (94). The key finding was that all techniques provide excellent diagnostic accuracy in detecting liver fibrosis in patients with NAFLD. MRE demonstrated the highest diagnostic accuracy for stage 4 detection and higher intra- and inter-observer reproducibility. The key points from a meta-analysis on the diagnostic performance of MRE in NAFLD are that MRE has high diagnostic accuracy for the staging of liver fibrosis in NAFLD (95). BMI does not significantly affect the accuracy of MRE in NAFLD (provided the patient can enter the MR bore to undergo the MRE examination), and inflammation had no significant influence on MRE performance in NAFLD for fibrosis. Inflammation may act as a confounder on liver fibrosis staging using traditional elastography techniques. There are at least three investigational techniques for assessing liver inflammation using MR: magnetic resonance viscoelastography, corrected T1, and diffusion-weighted imaging. Preliminary results in a dietary animal model of NASH suggest that the addition of a damping ratio (which is related to the ratio of viscosity over elasticity) increases with inflammation (96). Further studies in humans are needed to examine the exact utility and applicability of these approaches in the assessment of NAFLD severity. McDonald et al have proposed measuring the corrected T1 to detect the presence of inflammation (97). The rationale is that corrected T1 increases with the modified Ishak score but is also higher in the presence of inflammation but without fibrosis. Lefebvre et al have proposed a variant of diffusion-weighted imaging known as intravoxel incoherent motion to assess liver inflammation (98). Preliminary results indicate that perfusion fraction increases in patients with higher inflammation grades, providing classification accuracy ranging from 0.84 to 0.88 for non-invasive grading of inflammation. These techniques for assessing liver inflammation must be further validated in prospective, multicentre human cohorts.
This session closed with a debate on the important topic of screening to be done by primary care (pro versus con), featuring Prof Jeremy Cobbold (Oxford University Hospitals, Oxford, UK) and Prof Vlad Ratziu. Liver fibrosis screening in the primary care population is a significant health issue. Among the causes of liver fibrosis, NAFLD is not only highly prevalent but typically asymptomatic, and therefore most of the patients remain undiagnosed. Populations at risk for NAFLD-associated liver fibrosis, such as patients with T2D, are mostly seen in primary care and endocrinology clinics; as such, the development of clinical pathways to identify patients with advanced fibrosis is a key point for debate in NAFLD management.
Therapeutic approaches
The current therapeutic management of NASH relies on a three-tiered approach: lifestyle interventions, specific pharmacotherapy, and metabolic risk management. The guidelines recommend a program based on dietary change, weight loss, and structured exercise intervention. Pharmacotherapy should instead be reserved for patients with NASH and liver fibrosis. Given the strong and bidirectional association between NASH and T2D, there is emerging interest in pharmacotherapies used in T2D, with a need to address body weight. Several antifibrotic drugs are in phase II and phase III development through global clinical trials.
A healthy lifestyle, including diet and exercise for maintaining a healthy weight, is the cornerstone of NAFLD and NASH management (Figure 6). Before discussing nutritional treatment approaches, Dr Stéphanie Chevalier (McGill University, Montréal, Canada) stressed the importance of nutritional assessment. Although NAFLD and NASH are strongly associated with excess weight, patients may present with malnutrition or sarcopenia and should be screened, as both are predictors of morbidity and mortality (99). The Nutritional Risk Screening (2002) is a validated tool recommended for the malnutrition aspect (100), and sarcopenia (low muscle mass) may be assessed using dual X-ray absorptiometry or from abdominal cross-sectional CT images when available (101). In patients with overweight and obesity, an intensive lifestyle intervention leading to weight loss has shown the greatest benefits and is recommended as the first-line treatment (99). While a modest 5%–10% weight loss reduce steatosis, a more significant loss of >10% is needed for substantial fibrosis regression and NASH resolution (102). Few patients can achieve and maintain such loss in the long term. A multidisciplinary approach is recommended for sustainable lifestyle changes, which should include behaviour modification, education on weight cycling and hunger management, and self-monitoring in addition to physical activity and medications (103). Personalized hypo-energetic diets, irrespective of macronutrient composition, should be promoted for sustainability. Diets that are relatively high in protein are shown to preserve lean mass during weight loss (104) and combined with a low glycemic index to maintain weight after loss (105). While regular physical activity only achieves small amounts of weight loss, aerobic and resistance exercise have shown benefits in reducing visceral and hepatic fat even in the absence of weight loss, and on weight maintenance (106). Further, resistance training promotes the maintenance of muscle mass (107). Among different dietary patterns, the Mediterranean diet has been the most widely studied through observational and interventional studies and is recommended to reduce steatosis and improve insulin sensitivity (99). Similar plant-based dietary patterns should be further studied as they may lead to similar benefits. To date, only vitamin E supplements (800 IU alpha-tocopherol/d) are recommended, specifically in non-diabetic adults with histologically confirmed NASH for improvement of liver enzymes and histology (99). Although omega-3 fatty acid intake has shown benefits in reducing liver enzymes and steatosis, an optimal dose-response is unknown, and efficacy data are lacking to support a recommendation to take supplements. Likewise, antioxidant supplements are not recommended due to a lack of efficacy data. Given the potential involvement of the intestinal microbiome in NAFLD, supplements containing probiotics or synbiotics have been tested. Though data are limited, these may be used to improve liver enzymes (99). Regular coffee consumption (3–4 cups/d) was associated with reduced fibrosis in patients with NAFLD and is thought to most likely benefit, rather than harm. A high fructose intake, namely from beverages containing high fructose corn syrup, may contribute to NAFLD from the well-known role of fructose on de novo lipogenesis and potentially through an altered gut microbiome (108). Fructose may increase the risk of advanced fibrosis and NASH when in excess calories. Last, patients with NAFLD and NASH should abstain from alcohol to improve liver histology and reduce the risks of comorbidity (99). In conclusion, multimodal strategies are important and should include diet and lifestyle behaviours, medications, and bariatric surgery (as needed), as part of NAFLD and NASH treatment.
Figure 6:
Multimodal treatment strategies for NAFLD
Prof Quentin M Anstee (Newcastle University, Newcastle upon Tyne, UK) provided a detailed overview of the current management options for NAFLD. NAFLD prevalence increases in the obese (94%) and those with greater IR and T2D. As such, NAFLD is best thought of as part of a multisystem disease driven by obesity, which interlinks with metabolic stress, systemic inflammation, and fibrogenesis. Ideally, interventions should therefore be beneficial not only to the liver but also to other organ systems that may be impacted by the metabolic syndrome, such as the vasculature, heart, and kidney. Many factors contribute to today’s complex obesogenic environment, including physiologic, economic, social, cultural, and political factors. Thus, obesity and NAFLD are major societal challenges, and to truly address fatty liver disease, it is important to support individuals to make healthy lifestyle choices and develop personalized behaviour interventions. However, there is generally a poor public health readiness to respond to the challenges of NAFLD. A survey of 29 European countries showed that no participating country had written national strategies or a national action plan for NAFLD (109). There are four principles of treatment for targeting NAFLD: (1) target the obesogenic lifestyle by addressing obesity itself (dietary change and/or bariatric surgery) and sedentary behaviours (physical activity, exercise), (2) target the metabolic syndrome to reduce cardiovascular risk, ideally using agents with secondary liver-directed benefits, (3) target the liver itself by ameliorating steatohepatitis (and prevent progression to fibrosis and cirrhosis), and (4) minimize downstream complications such as HCC. The obesogenic Western diet is high in saturated fat, trans fat, salt, and refined ingredients as well as processed options (fast food), while it is low in plant-based options (60). There is evidence of the importance of weight loss to treat NAFLD. Weight reduction through a calorie restriction of a 500–1,000 kcal deficit per day should be recommended to patients with NAFLD as part of a holistic therapeutic approach because, if sustained, it improves cardiovascular risk profile, steatosis, steatohepatitis, and fibrosis (110). However, the intervention alone is not always effective: many individuals who attain these weight loss targets do not exhibit a complete histologic improvement. As such, there is a need to find better therapeutic options. Weight reduction should be recommended because, if sustained, it improves cardiovascular risk profile, steatosis, steatohepatitis (7%–9% weight loss), and fibrosis (>10% weight loss). One way to achieve this, which can be even better than simple calorie restriction, is the adoption of the Mediterranean diet—a diet that is high in planted-based foods, uses olive oil as the main source of added fat, and has moderate intakes of fish, seafood, eggs, white meat, and dairy products while including limited intakes of red/processed meats and sweets, and a moderate intake of red wine (102). An 18-month randomized controlled trial (RCT) with the Mediterranean diet versus an isocaloric low-fat diet in 278 sedentary adults with abdominal obesity or dyslipidemia showed a reduction in both weight and intrahepatic fat content (111). Another recent RCT showed that the Mediterranean diet, when supplemented with additional polyphenols, may have further benefits to reduce weight and hepatic fat content (112). Short-duration, very low-calorie diets can also have a beneficial effect on NAFLD by reducing weight and improving liver biochemistry (113). Bariatric surgery is the surgical equivalent of a very low-calorie diet. Indeed, weight loss through bariatric surgery, if sustained, leads to histologic improvements in all NASH components, including hepatocyte ballooning, lobular inflammation and steatohepatitis, although the improvement on fibrosis is more modest (114). Exercise also has beneficial effects in reducing liver fat content, as well as positive cardiovascular and psychological effects. Aerobic exercise at 150–300 minutes of moderate-to-vigorous-intensity >3 days per week has hepatic benefits. Resistance exercise 2–3 times per week improves insulin sensitivity and muscle strength/function. Vigorous-intensity aerobic exercise improves cardiorespiratory fitness and glycemic control (115). Many patients may need to be supervised and supported to introduce these changes, which need to be tailored to the individual patient due to frequent comorbidities. Of note, exercise alone does not adequately induce weight loss; while beneficial, it needs to be combined with a caloric restriction diet. It is important to integrate structured care delivery covering the major areas of management, including assessing the patient, looking for evidence of obesity, assessing comorbidities and how well the metabolic risk is being managed, and what lifestyle modifications are recommended. Partnering with patients also helps inform them such that they can make lifestyle changes. Delivering lifestyle changes encompasses helping the patient understand the NAFLD–lifestyle relationship, determining their weight status, exploring their dieting history, and assessing their readiness to change.
As for pharmacological therapy for NAFLD, there are currently no licensed medications for its treatment. Available drugs target either IR or oxidative stress. Considering agents that address IR first, there is little evidence that metformin is beneficial for NASH or fibrosis, although there are some data that it may reduce the HCC risk (116,117). Some phase II trial data supports the use of pioglitazone, liraglutide, and (more recently) semaglutide, although further studies are needed before firm recommendations can be made on the use of insulin-sensitizing agents to treat NAFLD in patients without T2D (118,119). Vitamin E targets oxidative stress. The PIVENS (Pioglitazone versus Vitamin E versus Placebo for the Treatment of Nondiabetic Patients with Nonalcoholic Steatohepatitis) study published in 2010 was a 96-week RCT of 247 non-diabetic non-cirrhotic adults with NASH randomized to either pioglitazone, vitamin E, or placebo (120). Both pioglitazone and vitamin E improved steatosis and inflammation, while only vitamin E reduced ballooning. Neither vitamin E nor pioglitazone reduced fibrosis. Based on these results, vitamin E has been increasingly used in North America but less so in Europe. Some subsequent studies in pre-diabetic and diabetic adults with NASH showed that pioglitazone resolved NASH but did not improve fibrosis when combined with a calorie-restricted diet (121). Safety and tolerability issues with vitamin E and pioglitazone should be acknowledged. Overall, these agents should be personalized for selected patients with histologically confirmed NASH after careful consideration of the risk/benefit ratio. The phase IIb LEAN (Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis) study, which included 45 patients with NASH randomized to either liraglutide or placebo for 48 weeks, showed that liraglutide improved NASH with no worsening of fibrosis (118). However, it was unclear to what extent these effects were entirely independent of weight loss. Recently, semaglutide also showed improvements in steatohepatitis but less in histologically assessed fibrosis (119). Overall, increasing evidence supports the use of glucagon-like peptide-1 (GLP-1) agonists in NASH, whether their effect is due to weight loss or a liver-specific effect.
In summary, the recommendations with currently available treatments include lifestyle change, treatment of the metabolic syndrome because more NAFLD patients will die from CVD than from liver cirrhosis. In this respect, it is also important to note that NAFLD does not compound the risk of statin-induced drug-induced liver injury, and as such, statin therapy should be actively considered to reduce the cardiovascular risk profile. Pioglitazone and GLP-1 agonists show some evidence of benefit for NASH, as does vitamin E and so these agents may be considered on an individual patient basis.
Prof Vlad Ratziu (Sorbonne University, Paris, France) discussed emerging therapy for advanced NAFLD (Figure 7). Unfortunately, there are several failed phase IIb and phase III trials for NASH and a recent success with the phase III trial of OCA (REGENERATE trial). The objective of NASH trials in non-cirrhotic patients is to prevent progression to cirrhosis to avoid associated deadly complications. It is important to select a population with a reasonable chance of progressing to cirrhosis: patients with steatohepatitis and some degree of fibrosis. These patients will also be eligible for novel pharmacotherapy once available on the market. For cirrhotic patients with NASH, the objectives are to prevent the occurrence of decompensation and to achieve fibrosis reversal (F ≤3), especially in those who have early cirrhosis. The difficulty is to select the right population; that is, whether those patients can reverse fibrosis or can progress to decompensation. If the wrong population is chosen, the effect during the trial will be missed. Selecting the right population is difficult because it is not known how to classify patients when they reach the cirrhotic stage in terms of both their remaining ability to have a reversal of fibrosis and their risk of progressing fast enough to decompensation within the trial. The classical indicators, such as Child–Pugh classification, MELD classification, portal pressure (HVPG), presence of varices, history of decompensation, and stiffness value are not accurate. The use of imperfect classification tools may explain why some trials fail: the right patients had not been selected. The hepatic events to be prevented in the cirrhotic population include gastrointestinal hemorrhage due to ruptured varices, ascites, encephalopathy, liver transplant. Significant biological changes may include a MELD increase from <12 to >15 and a Child–Pugh increase >2 points. It is also not known whether the occurrence of varices, or increase in their size, or HVPG reduction are sufficient indicators of a clinical event. Based on these considerations, the regulatory framework of phase III trials was based on two steps: the first step, histological improvement, is measured early within 1–1.5 years (if this step succeeds with ad interim analysis, the drug gets conditional approval); the second step is to continue the trial to assess if patients who improved histologically also achieve clinical benefits. The second step, the longer part of the study, implies continuing the trial with an outcome phase, long enough to measure clinical events or progression to cirrhosis (if this step succeeds, the drug receives definitive approval). During the second step of the trial, there are major issues pertaining to patients’ retainment and being on placebo for a long period. In the REGENERATE study (non-cirrhotic NASH), 2,200 patients from 23 countries were enrolled (122). The first analysis, at 18 months, was performed in almost 1,000 patients, and OCA had a positive dose-response effect on improving fibrosis (reversal of fibrosis by one stage or more) in 23% patients versus 12% in patients on placebo. These results confirm the phase II trial, making this drug the only one that achieved this level of demonstration. The issue is not only how many people have a reversal of fibrosis but also how many people do not progress. With OCA, there were more people improving fibrosis and fewer people worsening fibrosis. In the high dose arm, there was a three-fold higher probability of having an improvement of fibrosis than a worsening, which likely means a real antifibrotic effect. When looking at two-stages fibrosis improvement, the response was lower, but still significant, in the active arm compared to placebo. The resolution of NASH was not formally achieved in the analysis, but OCA may be able to reduce steatohepatitis since, in the high dose arm, ballooning and lobular inflammation improved. In the phase II trial, a robust decrease in ALT at week 24 was a good predictor of histological response, so this finding might help predict who will respond (123). In terms of safety and tolerability, OCA causes pruritis and lipid abnormalities. The FXR agonists fibroblast growth factor 19 (FGF19) impact on lipids, with an increase in LDL cholesterol, a reduction in HDL, and a reduction in triglycerides. The effect seems dependent on the dose of OCA. A way to control this undesirable effect is to use statins. A question to consider is whether this increase in LDL translates to increased cardiovascular risk. Some drugs developed for NASH target specifically patients with liver cirrhosis. REVERSE (Randomized Phase 3 Study Evaluating the Efficacy and Safety of Obeticholic Acid in Subjects with Compensated Cirrhosis Due to Nonalcoholic Steatohepatitis) is a 2-year trial of OCA in patients with compensated NASH cirrhosis with both histological end point (reversal of fibrosis) and a clinical end point (clinical events). Second-generation FXR agonists are in development that would potentially have fewer side effects than OCA while preserving the same efficacy. Among them, the cilofexor was reported to have less potency due to pharmacokinetics reasons, although pruritus and dyslipidemia were less frequent (124). Biochemical response in terms of liver transaminases was not significant and mainly occurred in patients with a decrease in MRI-PDFF. Tropifexor is another second-generation FXR agonist. The phase IIb trial showed no effect on NASH resolution or fibrosis improvement, although there was a positive reduction in collagen deposits within septa. In a phase IIa trial, EDP-305 showed a strong effect in terms of engaging the target, inducing FGF19, and reducing biliary excretion. EDP-305 reduced both MRI-PDFF and ALT, but it produced an increase of LDL, although of lower magnitude than OCA, and pruritus in almost 50% of patients. Overall, all FXR agonists have the same side effects; as such, it is important to find the right dose with histological efficacy but no tolerability issues. FGF19 drugs modify the FGF19 molecule to retain the bile acid effect without inducing the tumours described in rodents. Aldafermin retains the FGF19 bile acid effects but loses the tumorigenic effects. A 6-month phase II trial of aldafermin showed a potent reduction in bile acid synthesis, liver fat, and ALT (125). Despite the small numbers, the study showed histological benefits (22% versus 0% in placebo) for a composite outcome of fibrosis improvement and NASH resolution. As a side effect, there is a strong increase in LDL, which could be controlled with a statin. Another drug that showed positive effects is lanifibranor, a pan peroxisome proliferator-activated receptor agonist. Although the alpha, delta, or gamma effect is relatively weak, when all three subtypes are targeted, there are significant benefits in terms of improving inflammation and fibrosis compared to dual agonists (126). In a 6-month phase II trial of 247 patients, lanifibranor showed significant resolution of NASH and fibrosis improvement. GLP-1 receptor agonists like semaglutide have a clear central effect, but there is no clear hepatic effect. The liver effect is carried through weight loss due to central satiety induction (119). GLP-1 agonists are more complicated drugs being injectable and requiring dose escalation to avoid side effects. However, they induce important weight loss with consequent histological improvement (59% resolution of NASH versus 17% in placebo, but interestingly no dose-response). There was also ALT improvement, a 12% decrease in body weight, and a strong effect on hemoglobin glycated. Although this was an 18-month study, it did not demonstrate an improvement in fibrosis, but it may take longer to show an effect on fibrosis. GLP-1 glucose-dependent insulinotropic polypeptide dual agonists may achieve an even higher rate of weight loss than semaglutide, which likely will have an added benefit for histological improvement (127). Aramchol is an inhibitor of steroyl-CoA desaturase-1, a key enzyme in hepatic lipogenesis that converts saturated fatty acids into monounsaturated fatty acids. Preclinical models show that inhibition of steroyl-CoA desaturase-1 results in less weight gain, reduced IR, less steatosis. In a phase IIb trial, aramchol showed significant ALT reduction, and a signal for improvement in fibrosis and NASH resolution (128). Resmetirom is an oral, liver-directed, first in class thyroid hormone agonist beta selective agonist which lowers LDL cholesterol, triglycerides, liver fat, potentially reducing lipotoxicity and NASH. The phase II trial showed strong antisteatogenic effect, with many patients achieving the threshold of more than 30% liver fat reduction, reduction in ALT, steatohepatitis resolution (129). There was also improvement in lipid profile, while no effect on the glycemic side.
Figure 7:
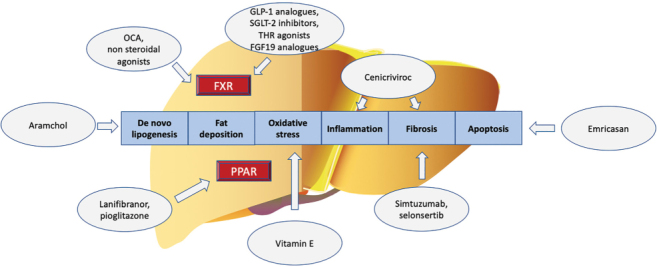
Targets of NASH therapy
NASH = Non-alcoholic steatohepatitis; OCA = Obeticholic acid; FXR = Farnesoid X receptors; FGF19 = Fibroblast growth factor 19; GLP-1 = Glucagon-like peptide 1; SGLT-2 = Sodium-glucose transport protein 2; THR = Thyroid hormone receptor; PPAR = Peroxisome proliferator-activated receptors
Metabolic syndrome and CVD
Heather Watson provided a heart-warming patient perspective of her journey to liver transplantation. Retrospectively, she recognized that the journey of her liver disease truly started in her youth and with a history of obesity. Just prior to age 40 years, she started feeling unwell with non-specific aspects of fatigue, which can often be overlooked. It was presumed that her busy lifestyle as a working mother with young children was responsible for her fatigue and general unwellness. There was no consideration that this may have been due to liver disease. It was not until Heather was admitted to hospital with a suspicion of gallstone disease that, based on lab-findings, she was diagnosed with liver disease. This was believed to be the underlying issue secondary to NASH. Heather was not aware that NASH even existed up to this point. This situation did highlight the lack of overall awareness of NASH and the importance of increasing awareness in the public domain and the medical field. A liver biopsy diagnosed advanced fibrosis. Therefore, Heather was provided with a new diagnosis of NASH and informed that she also had an advanced disease—all before the age of 40 years! Heather described feeling overwhelmed as she continued to feel unwell. There was a recognition of the uncontrollable progressive liver disease and an increasing MELD score, with consideration of liver transplantation as the only long-term option. She described her challenges with the additive symptoms of decompensated disease, which required weekly paracentesis and an increasing need for medical care. Heather recognized her own challenges, but also those for her family and friends. She described the challenges of staying in hospital, with complications of nausea, epistaxis, encephalopathy; as well, she had already developed diuretic-resistant ascites. Heather described in a very touching manner saying farewell to family, including her father, children, and siblings, along with the challenges that would continue in her poor health. She described aspects of losing hope as she was so unwell—in particular, a touching discussion with her father and a conversation with her children about her not returning home from the hospital.
Incredibly, that very day she was informed that a liver transplant would be available. This was a highly emotional time of joy given the potential for a transplant, but she also recognized that her family was suffering through the process and further, that the donor family was suffering from a significant loss. A surreal mix of joy and sorrow and extremes of emotion were made more challenging by the effects of encephalopathy on her memories.
The journey continues, as Heather describes the importance of post-transplant rehabilitation and the effort to return to skills of “regular living.” Heather describes, in conclusion, the continued challenges of understanding the root cause of weight issues and the overall lack of people’s recognition of the same. She also underlines her gratitude to the donor family and the medical community for a second chance at life. Heather’s journey advocates for awareness of NASH, given the lack of recognition of this disease state and the risk factors in the general population—and to some degree, even in the medical field. Others should understand her story to try and affect a positive change and, in particular, to be evaluated early.
Obesity and NAFLD are linked through IR, with metabolic syndrome as a central theme. Central obesity is a key aspect and linked to metabolic syndrome with the development of T2D. Indeed, over 80% of NASH patients suffer from obesity, and 40% from T2D. These are converging themes with the commonality of metabolic syndrome. Dr Harpreet Bajaj (LMC Healthcare, University of Toronto, Toronto, Ontario, Canada) suggests considering other end-organ aspects, including sleep apnea, coronary artery disease, hypertension, T2D, nephropathy, dyslipidemia, and osteoporosis (130). The pathogenesis of metabolic syndrome is likely related to IR. This may be secondary to high calorie intake, particularly high fructose foods, and excess saturated fats, coupled with a sedentary lifestyle, leading to obesity, T2D, and NAFLD (131). An alternate theory is the “Twin Cycle” hypothesis, with ectopic deposition of fat in the pancreas and liver, leading to T2D and NAFLD (132). Complications of T2D can be classified as microvascular, microvascular, and other non-classical ones to include dementia, NAFLD, sleep apnea, and depression, which are often not recognized. Thus, one must think from a more diverse perspective and include these additional complications seen in T2D. There is a clear increased risk of NAFLD by two- to three-fold in persons with T2D, increased progression of liver disease, and development of cirrhosis with an increased risk of HCC (133,134). The National Health and Nutrition Examination Survey (NHANES) dataset shows that T2D is the strongest predictor of progression of NASH. At present, there are no clear consensus guidelines on screening for NAFLD in T2D; further, the modality used for diagnosis also needs to be clarified (135). Investigations to diagnose NAFLD may include biochemical or imaging options, for which we would need to consider availability and cost-effectiveness. Case-finding may be reasonable given the higher prevalence of NAFLD in T2D and the increased morbidity and mortality. Therapeutic options for NASH are challenging, with no specific pharmacological option available. Presently, lifestyle changes and weight loss should be a mainstay. However, the challenge of lifestyle modifications is the maintenance of weight loss. One should aim for 10% weight loss and maintain this for 52 weeks, but a low proportion (10%) of persons can achieve this. When sustained weight loss is achieved, there is improvement in NASH steatosis, inflammation, and fibrosis (102). Dr Bajaj discussed an initiative with the Canadian Diabetes Prevention Program, a pilot study with online coaching to enrol 2,000 persons (see www.LMC.ca/diabetes-prevention@lmc.ca).
Pharmacological agents used to treat T2D may benefit liver disease. There are ongoing clinical trials with GLP-1 agonists and sodium-dependent glucose cotransporters 2 (SGLT-2) inhibitors. There is a known significant benefit on weight loss with GLP-1 agonists with improvement in NASH (119). Presently, the ESSENCE trial for semaglutide for F2–3 fibrosis is ongoing. More potent SGLT2 inhibitors such as tirzepatide are used in phase II studies, such as the SYNERGY study (clinicaltrials.gov NCT04166773). SGLT2 inhibitors can result in increased ALT reduction independent of weight loss (136). The evolution of treatment in T2D will likely lead to more individualized targets. Agents initially aimed at glycemic control, such as GLP-1 agonists and SGLT2 inhibitors, also aim to improve cardiovascular and renal end points in high-risk subpopulations. This evolution may have similarities with NAFLD. The overall approach should include individualization of care, utilization of a multidisciplinary team (to include primary care and metabolism specialists earlier in the disease), and consideration of future combination pharmacologic therapy.
Overall, an increasing proportion of people with NAFLD are affected by coronary artery disease, with NAFLD being an independent risk factor for it. Notably, the severity of coronary artery disease is increased in proportion to the severity of NAFLD (137). There is a role for systemic inflammation in the pathogenesis of coronary artery disease and NAFLD. Data suggest that NAFLD may be a driver of hypertension, and the pathophysiology may be secondary to systemic inflammation, IR, effects of oxidative stressors, and vasoconstriction (138). There are various CVD associations with NAFLD. Dr James Stone (University of Calgary, Canada) discussed the concept of considering orders of magnitude when discussing associations of conditions. The prevalence of obesity is linked to NAFLD and CVD. Patients with NAFLD are five-fold more likely to develop T2D, compared to those without T2D (139). Serum cholesterol, including a higher LDL cholesterol in the normal range, increases the risk of NAFLD (140). In essence, the severity of liver disease affects the severity of CVD risk factors, and vice versa. The order of magnitude in associations is important when considering the various risk factors described. There is an association between increased CVD mortality and increasing severity of NAFLD, which is correlated to increasing level of steatosis and mortality (141). In persons with NASH, increasing fibrosis is correlated with an increased risk of all-cause mortality (8). Overall, severity is a good predictor of prognosis. In a prospective cohort study, NAFLD persons had a two-fold increased risk of CVD, and if fibrosis is present, they would have a four-fold increased risk of CVD (142). There may be a debate between NAFLD and CVD outcomes—who is the driver and who is the passenger? In essence, it is one and the same, as the natural history is similar. The underlying systemic inflammation and alteration in vascular milieu—with overall pathogenesis linked to the metabolic syndrome—is similar. There are several common CVD complications noted in persons with NAFLD. Heart failure is more common in NAFLD due to its relationship to cardiomyopathies; that is, increasing hepatic steatosis is linked to increased diastolic dysfunction (30). Dysrhythmias are also more frequently noted, with atrial fibrillation three-fold higher in people with NAFLD (143). Ventricular ectopy is also more common in those with NAFLD. Coronary artery disease is more frequent in persons with NAFLD and increases in severity in those with more advanced NAFLD (144).
Various therapeutic strategies can be considered in people with NAFLD. Suggested health behaviour modifications generally include nutritional and weight management, physical activity, and attention to mental health. Specifically, caloric restriction is suggested, including low carbohydrate diets, Mediterranean diets, and intermittent fasting. A person would need a sustained 10% reduction in body weight to reduce inflammation and fibrosis in NAFLD (102). Physical activity can also be effective and should be initiated. Mental health may also be affected by a systemic inflammatory process. People with NAFLD do have increased rates of depression and anxiety, suggesting the possibility of a similar pathway (145).
In summary, NAFLD and CVD are both chronic inflammatory diseases with a pathogenesis of systemic inflammation. Persons with NAFLD are very likely to have CVD and vice versa. A dynamic debate entitled “Why do I care about NAFLD if patients will suffer heart disease (heart versus liver)?” closed the session by featuring Dr James Stone for heart and Dr Alnoor Ramji (University of British Columbia, Vancouver, Canada) for liver.
Outcomes of the 1st International Workshop of CanNASH
The 1st International Workshop of CanNASH was an interdisciplinary meeting of scientists, clinicians, and community members. The pathophysiology and complications session presentations described the complexity of the fibrogenetic process driving NASH pathogenesis, which should be considered for developing therapeutic targets. Moreover, it clearly demonstrated the different natural history of the disease compared to other etiologies of liver disease and the need for dedicated, large-scale studies to answer open questions on complications and outcomes. Finally, it underlined the importance of considering NASH as a multisystem disease, where extrahepatic manifestations should be contemplated for clinical management and comprehensive therapeutic strategies. The diagnostics and biomarkers presentations demonstrated that liver biopsy is still required for a definitive diagnosis of NASH, although non-invasive tests can help identify patients with significant liver fibrosis.
Moreover, non-invasive tests could help develop referral pathways to hepatology by identifying patients at risk of having advanced liver fibrosis. The therapeutic management session showed that treatment of NASH relies on three essential components: lifestyle interventions, specific pharmacotherapy, and metabolic risk management. While weight loss can achieve significant fibrosis regression, the optimal dietary approach and the optimization of sustained weight loss, remain not well established. Moreover, pharmacotherapy is still suboptimal, while several therapeutic targets are being developed in global clinical trials. Given the strong and bidirectional association between NASH and T2D, pharmacotherapies used in T2D will likely become of more common use in the future. This meeting and CanNASH are committed to putting Canada at the forefront of research and policy changes by contributing to the global effort into NASH as a silent killer.
Funding Statement
G Sebastiani is supported by a Senior Salary Award from FRQS (#296306).
Ethics Approval:
N/A
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
The 1st International Workshop of CanNASH joint event—with the single topic conference of CASL—was supported by a Canadian Institutes of Health Research (CIHR) research grant (#429609), by Réseau SIDA, Fonds de la Recherche en Santé du Quebéc (FRQS), by the Cardiometabolic Health, Diabetes and Obesity Research Network of FRQS, and both the Research Institute (RI–MUHC) and the Infectious Diseases and Immunity in Global Health Program (IDIGH) at the McGill University Health Centre. Additional funding was provided by Intercept, Novo Nordisk, Pfizer, AstraZeneca, Lilly, Gilead, Novartis, Thera Technologies, Bristol Meyers Squibb, and Merck. The views expressed in this publication are those of the authors and do not reflect the position of the CIHR, FRQS, or other sources of funding. G Sebastiani is supported by a Senior Salary Award from FRQS (#296306).
Disclosures:
G Sebastiani has acted as a speaker for Pfizer, Merck, Novo Nordisk, Novartis, Gilead, and AbbVie, served as an advisory board member for Merck, Gilead, Pfizer, Allergan, Novo Nordisk, Intercept Pharmaceuticals, and Novartis, and has received research funding from Merck and Theratec. K Patel is a speaker for Gilead Sciences, Intercept, and Abbott, an advisory board/consultant for Gilead Sciences, Intercept, Novartis, and Allergan, and has received research funding from Gilead Sciences. V Ratziu consults for Genfit, Intercept, Galmed, Boehringer Ingelheim, AstraZeneca, Bristol-Myers Squibb, and Madrigal. He has received grants from Gilead. JJ Feld is a consultant/advisor and/or has received research support from AbbVie, Arbutus, Antios, Enanta, Eiger, Finch, Gilead Sciences, GSK, Janssen, and Roche. BA Neuschwander-Tetri is a consultant for Allergan, Allysta, Arrowhead, ARTham, Axcella, Blade, Boehringer Ingelheim, BMS, Coherus, Consynance, Durect, Enanta, Fortress, Gelesis, Gilead, HistoIndex, Intercept, Lipocine, Madrigal, Medimmune, Merck, Metacrine, Mundipharma, NGM, pH-Pharma, Prometheus, and Siemens. He has received institutional research grants from Allergan, BMS, Cirius, CymaBay, Enanta, Galectin, Genfit, Gilead, Intercept, Madrigal, NGM, and Prometheus. M Pinzani is an advisory board/consultant for Promethera (Belgium), Chemomab (Israel), Takeda (USA), LimmaTech Biologics (Switzerland), Resolution Therapeutics (UK), Dicerna (USA), Astra Zeneca (UK). He is a co-founder/director and shareholder of Engitix Therapeutics Ltd (UK) and 3P-Sense Ltd (UK). He is Chief Medical Officer of Hepatotargets/BitBio Ltd (UK). S Petta has been a speaker and/or advisor for AbbVie, Gilead, and Intercept. A Berzigotti has been an advisory board member for Inventiva, Bracco, General Electrics, and Boehringer Ingelheim. EM Brunt has been a study pathologist for CymaBay, Histoindex, and NGM, a consultant for Alnylam/Regeneron Therapeutics, Arrowhead Pharmaceutical, and Perspectum Diagnostics, and a member of the advisory board for Pfizer. A Tang is a speaker for Eli Lilly and has received research support from Siemens. JF Cobbold has received consultancy fees from Novo Nordisk and speaker fees from Intercept. P Ghali has been a consultant for Merck and Gilead. QM Anstee reports grants from AbbVie, Allergan/Tobira, AstraZeneca, GlaxoSmithKline, Glympse Bio, Novartis Pharma AG, Pfizer Ltd., and Vertex. He also reports consultancy for 89Bio, Abbott Laboratories, Acuitas Medical, Allergan/Tobira, Altimmune, AstraZeneca, Axcella, Blade, BMS, BNN Cardio, Celgene, Cirius, CymaBay, EcoR1, E3Bio, Eli Lilly & Company Ltd., Galmed, Genentech, Genfit SA, Gilead, Grunthal, HistoIndex, Indalo, Imperial Innovations, Intercept Pharma Europe Ltd., Inventiva, IQVIA, Janssen, Madrigal, MedImmune, Metacrine, NewGene, NGMBio, North Sea Therapeutics, Novartis, Novo Nordisk A/S, PathAI, Pfizer Ltd., Poxel, ProSciento, Raptor Pharma, Servier, Terns, and Viking Therapeutics. He is a speaker for Abbott Laboratories, Allergan/Tobira, BMS, Clinical Care Options, Falk, Fishawack, Genfit SA, Gilead, Integritas Communications, Kenes, and MedScape. He further reports royalties from Elsevier Ltd. H Bajaj reports receiving grant support from Amgen, Kowa, and Sanofi US Services, and grant support and lecture fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen Global Services, and Novo Nordisk. MG Swain served on an advisory board and speakers bureaus for Gilead, Intercept, Abbott, Novartis. He has also received clinical trial funding from Gilead, Intercept, CymaBay, Genkyotex, GSK, and Novartis. A Ramji served on advisory boards and speakers bureaus for Gilead, Intercept, AbbVie, Celgene, Merck, and Novartis. He has also received clinical trial funding AbbVie, Assembly, Gilead, Intercept, Galmed, Janssen, Springbanks, Allergan, and Novartis. The other coauthors have nothing to disclose related to this publication.
Peer Review:
This article has been peer reviewed.
Animal Studies:
N/A
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. 10.1002/hep.28431. Medline: [DOI] [PubMed] [Google Scholar]
- 2.Swain MG, Ramji A, Patel K, et al. Burden of nonalcoholic fatty liver disease in Canada, 2019–2030: a modelling study. CMAJ Open. 2020;8(2):E429–36. 10.9778/cmajo.20190212. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sebastiani G, Ramji A, Swain MG; Patel K. A Canadian survey on knowledge of non-alcoholic fatty liver disease among physicians. Can Liv J. 2021;4(2):82–92. 10.3138/canlivj-2020-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;6(1)7:328–57. 10.1002/hep.29367. Medline: [DOI] [PubMed] [Google Scholar]
- 5.Wong RJ, Aguilar M, Cheung R, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the U.S. Gastroenterol. 2015;148(3):547–55. Epub 2014 Nov 25. 10.1053/j.gastro.2014.11.039. Medline: [DOI] [PubMed] [Google Scholar]
- 6.Noureddin M, Vipani A, Bresee C, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113(11):1649–59. 10.1038/s41395-018-0088-6. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic steatohepatitis is the most rapidly increasing indication for liver transplantation in the United States. Clin Gastroenterol Hepatol. 2021;19(3):580–9.e5. 10.1016/j.cgh.2020.05.064. Medline: [DOI] [PubMed] [Google Scholar]
- 8.Hagstrom H, Nasr P, Ekstedt M, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67(6):1265–73. 10.1016/j.jhep.2017.07.027. Medline: [DOI] [PubMed] [Google Scholar]
- 9.Estes C, Razavi H, Loomba R, Younossi Z, Sanyal AJ. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. 10.1002/hep.29466. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–23. 10.1126/science.1204265. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neuschwander-Tetri BA. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–88. 10.1002/hep.23719. Medline: [DOI] [PubMed] [Google Scholar]
- 12.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–5. 10.1038/ng.257. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–25.e716. 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Unger RH, Clark GO, Scherer PE, Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta. 2010;1801(3):209–14. 10.1016/j.bbalip.2009.10.006. Medline: [DOI] [PubMed] [Google Scholar]
- 15.Brunt EM, Wong VW, Nobili V, et al. Nonalcoholic fatty liver disease. Nat Rev Dis Primers. 2015;1: Article 15080. 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 16.Geng Y, Faber KN, de Meijer VE, Blokzijl H, Moshage H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol Int. 2021;15(1):21–35. 10.1007/s12072-020-10121-2. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57(10): 1758–70. 10.1194/jlr.R066357. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musso G, Cassader M, Paschetta E, Gambino R. Bioactive lipid species and metabolic pathways in progression and resolution of nonalcoholic steatohepatitis. Gastroenterology. 2018;155(2):282–302.e288. 10.1053/j.gastro.2018.06.031. Medline: [DOI] [PubMed] [Google Scholar]
- 19.Marra F, Svegliati-Baroni G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J Hepatol. 2018;68(2):280–95. 10.1016/j.jhep.2017.11.014. Medline: [DOI] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri BA. Therapeutic landscape for NAFLD in 2020. Gastroenterology. 2020;158(7):1984–98.e1983. 10.1053/j.gastro.2020.01.051. Medline: [DOI] [PubMed] [Google Scholar]
- 21.Parola M, Robino G, Marra F, et al. HNE interacts directly with JNK isoforms in human hepatic stellate cells. J Clin Invest. 1998;102(11): 1942–50. 10.1172/JCI1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pinzani M, Macias-Barragan J. Update on the pathophysiology of liver fibrosis. Expert Rev Gastroenterol Hepatol. 2010;4(4):459–72. 10.1586/egh.10.47. Medline: [DOI] [PubMed] [Google Scholar]
- 23.Liu YL, Patman GL, Leathart JB, et al. Carriage of the PNPLA3 rs738409 C>G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61(1):75–81. 10.1016/j.jhep.2014.02.030. Medline: [DOI] [PubMed] [Google Scholar]
- 24.Bruschi FV, Claudel T, Tardelli M, et al. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatolog 2017;65(6):1875–90. 10.1002/hep.29041. Medline: [DOI] [PubMed] [Google Scholar]
- 25.Eslam M, Sanyal AJ, George J; International Consensus Panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. 10.1053/j.gastro.2019.11.312. Medline: [DOI] [PubMed] [Google Scholar]
- 26.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–97.e10. 10.1053/j.gastro.2015.04.043. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D, Adejumo AC, Yoo ER, et al. Trends in mortality from extrahepatic complications in patients with chronic liver disease, from 2007 through 2017. Gastroenterology. 2019; 157(4):1055–66.e11. 10.1053/j.gastro.2019.06.026. Medline: [DOI] [PubMed] [Google Scholar]
- 28.Paik JM, Golabi P, Biswas R, Alqahtani S, Venkatesan C, Younossi ZM. Nonalcoholic fatty liver disease and alcoholic liver disease are major drivers of liver mortality in the United States. Hepatol Commun. 2020;4(6):890–903. 10.1002/hep4.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mantovani A, Byrne CD, Bonora E, Targher G. Nonalcoholic fatty liver disease and risk of incident type 2 diabetes: a meta-analysis. Diabetes Care. 2018;41(2):372–82. 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 30.Bonapace S, Perseghin G, Molon G, et al. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–95. 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pais R, Giral P, Khan JF, et al. Fatty liver is an independent predictor of early carotid atherosclerosis. J Hepatol. 2016;65(1):95–102. 10.1016/j.jhep.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 32.Dongiovanni P, Petta S, Maglio C, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61(2):506–14. 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 33.Targher G, Byrne CD, Lonardo A, Zoppini G, Barbui C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J Hepatol. 2016;65(3):589–600. 10.1016/j.jhep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Kim GA, Lee HC, Choe J, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol. 2017;68(1):140–6. 10.1016/j.jhep.2017.09.012. Medline: [DOI] [PubMed] [Google Scholar]
- 35.Ma J, Hwang SJ, Pedley A, et al. Bi-directional analysis between fatty liver and cardiovascular disease risk factors. J Hepatol. 2017;66(2):390–7. 10.1016/j.jhep.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y, Jung HS, Yun KE, Cho J, Cho YK, Ryu S. Cohort study of non-alcoholic fatty liver disease, NAFLD fibrosis score, and the risk of incident diabetes in a Korean population. Am J Gastroenterol. 2013;108(12):1861–8. 10.1038/ajg.2013.349. [DOI] [PubMed] [Google Scholar]
- 37.Ampuero J, Aller R, Gallego-Duran R, et al. Significant fibrosis predicts new-onset diabetes mellitus and arterial hypertension in patients with NASH. J Hepatol. 2020; 73(1):17–25. 10.1016/j.jhep.2020.02.028. [DOI] [PubMed] [Google Scholar]
- 38.Targher G, Bertolini L, Padovani R, et al. Relations between carotid artery wall thickness and liver histology in subjects with nonalcoholic fatty liver disease. Diabetes Care. 2006;29(6):1325–30. 10.2337/dc06-0135. [DOI] [PubMed] [Google Scholar]
- 39.Petta S, Argano C, Colomba D, et al. Epicardial fat, cardiac geometry and cardiac function in patients with non-alcoholic fatty liver disease: association with the severity of liver disease. J Hepatol. 2015;62(4):928–33. 10.1016/j.jhep.2014.11.030. [DOI] [PubMed] [Google Scholar]
- 40.Vilar-Gomez E, Sookoian S, Pirola CJ, et al. ADH1B *2 is associated with reduced severity of nonalcoholic fatty liver disease in adults, independent of alcohol consumption. Gastroenterology. 2020;159(3):929–43. 10.1053/j.gastro.2020.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43(4): 682–9. 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 42.Sanyal AJ, Harrison SA, Ratziu V, et al. The natural history of advanced fibrosis due to nonalcoholic steatohepatitis: data from the Simtuzumab trials. Hepatology. 2019;70(6):1913–27. 10.1002/hep.30664. [DOI] [PubMed] [Google Scholar]
- 43.Groszmann RJ, Garcia-Tsao G, Bosch J, et al. Beta-blockers to prevent gastroesophageal varices in patients with cirrhosis. N Engl J Med. 2005;353:2254–61. 10.1056/NEJMoa044456. [DOI] [PubMed] [Google Scholar]
- 44.Berzigotti A, Garcia-Tsao G, Bosch J, et al. Obesity is an independent risk factor for clinical decompensation in patients with cirrhosis. Hepatology. 2011;54(2):555–61. 10.1002/hep.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sourianarayanane A, Talluri J, Humar A, McCullough AJ. Stage of fibrosis and portal pressure correlation in nonalcoholic steatohepatitis. Eur J Gastroenterol Hepatol. 2017;29(5):516–23. 10.1097/MEG.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 46.Ferrusquia-Acosta J, Bassegoda O, Turco L, et al. Agreement between wedged hepatic venous pressure and portal pressure in non-alcoholic steatohepatitis-related cirrhosis. J Hepatol. 2021;74(4):811–18. 10.1016/j.jhep.2020.10.003. [DOI] [PubMed] [Google Scholar]
- 47.Mendoza YP, Shengir M, Bosch J, Sebastiani G, Berzigotti A. FIB-4 improves LSM-based prediction of complications in overweight or obese patients with compensated advanced chronic liver disease. Clin Gastroenterol Hepatol. 2021;S1542-3565(21):00271–8. Epub 2021 Mar 25. https://dpi.org/10.1016/j.cgh.2021.03.007. Medline: [DOI] [PubMed] [Google Scholar]
- 48.Petta S, Sebastiani G, Bugianesi E, et al. Non-invasive prediction of esophageal varices by stiffness and platelet in non-alcoholic fatty liver disease cirrhosis. J Hepatol. 2018;69(4):878–85. 10.1016/j.jhep.2018.05.019. [DOI] [PubMed] [Google Scholar]
- 49.Stine JG, Shah NL, Argo CK, Pelletier SJ, Caldwell SH, Northup PG. Increased risk of portal vein thrombosis in patients with cirrhosis due to nonalcoholic steatohepatitis. Liver Transpl. 2015;21(8):1016–21. 10.1002/lt.24134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodrigues SG, Brabandt B, Stirnimann G, Maurer MH, Berzigotti A. Adipopenia correlates with higher portal pressure in patients with cirrhosis. Liver Int. 2019;39(9):1672–81. 10.1111/liv.14175. [DOI] [PubMed] [Google Scholar]
- 51.Ge J, Lai JC, Boike JR, et al. Nonalcoholic fatty liver disease and diabetes mellitus are associated with post-transjugular intrahepatic portosystemic shunt renal dysfunction: an advancing liver therapeutic approaches group study. Liver Transpl. 2021;27(3):329–40. 10.1002/lt.25949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Montano-Loza AJ, Angulo P, Meza-Junco J, et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–35. 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Younossi ZM, Otgonsuren M, Henry L, et al. Association of nonalcoholic fatty liver disease (NAFLD) with hepatocellular carcinoma (HCC) in the United States from 2004 to 2009. Hepatology. 2015;62(6):1723–30. 10.1002/hep.28123. [DOI] [PubMed] [Google Scholar]
- 54.Younossi Z, Stepanova M, Ong JP, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17(4):748–55.e743. 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 55.D'Avola D, Labgaa I, Villanueva A. Natural history of nonalcoholic steatohepatitis/nonalcoholic fatty liver disease-hepatocellular carcinoma: magnitude of the problem from a hepatology clinic perspective. Clin Liver Dis (Hoboken). 2016;8(4):100–4. 10.1002/cld.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mittal S, El-Serag HB, Sada YH, et al. Hepatocellular carcinoma in the absence of cirrhosis in United States veterans is associated with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2016;14(1):124–31.e1. 10.1016/j.cgh.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Piscaglia F, Svegliati-Baroni G, Barchetti A, et al. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: a multicenter prospective study. Hepatology. 2016;63(3):827–38. 10.1002/hep.28368. [DOI] [PubMed] [Google Scholar]
- 58.Nair S, Mason A, Eason J, Loss G, Perrillo RP. Is obesity an independent risk factor for hepatocellular carcinoma in cirrhosis? Hepatology. 2002;36(1):150–5. 10.1053/jhep.2002.33713. [DOI] [PubMed] [Google Scholar]
- 59.Yang JD, Ahmed F, Mara KC, et al. Diabetes is associated with increased risk of hepatocellular carcinoma in patients with cirrhosis from nonalcoholic fatty liver disease. Hepatology. 2020;71(3):907–16. 10.1002/hep.30858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease. J Hepatol 2016;64:1388–1402. 10.1016/j.jhep.2015.11.004. Medline: [DOI] [PubMed] [Google Scholar]
- 61.Myers RP, Fong A, Shaheen AA. Utilization rates, complications and costs of percutaneous liver biopsy: a population-based study including 4275 biopsies. Liver Int. 2008;28(5):705–12. 10.1111/j.1478-3231.2008.01691.x. Medline: [DOI] [PubMed] [Google Scholar]
- 62.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology. 2009;49(3):1017–44. 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 63.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(5):1264–81.e1264. 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41(6):1313–21. 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 65.Brunt EM, Kleiner DE, Wilson LA, Belt P, Neuschwander-Tetri BA. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011; 53(3):810–20. 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Skelly MM, James PD, Ryder SD. Findings on liver biopsy to investigate abnormal liver function tests in the absence of diagnostic serology. J Hepatol. 2001;35(2):195–9. 10.1016/S0168-8278(01)00094-0. [DOI] [PubMed] [Google Scholar]
- 67.Brunt EM. Alcoholic and nonalcoholic steatohepatitis. Clin Liver Dis. 2002;6(2): 399–420. 10.1016/S1089-3261(02)00002-8. [DOI] [PubMed] [Google Scholar]
- 68.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol. 2015;13(4):643–54. 10.1016/j.cgh.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brunt EM, Kleiner DE, Wilson LA, Sanyal AJ, Neuschwander-Tetri BA; Nonalcoholic Steatohepatitis Clinical Research Network. Improvements in histologic features and diagnosis associated with improvement in fibrosis in nonalcoholic steatohepatitis: results from the Nonalcoholic Steatohepatitis Clinical Research Network treatment trials. Hepatology. 2019;70(2):522–31. 10.1002/hep.30418. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73(6):1322–32. 10.1016/j.jhep.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 71.Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase IIb study. J Hepatol. 2020;72(4):613–26. 10.1016/j.jhep.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 72.Matthews Brunt E. Liver biopsy reliability in clinical trials: thoughts from a liver pathologist. J Hepatol. 2020;73(6):1310–12. 10.1016/j.jhep.2020.08.014. [DOI] [PubMed] [Google Scholar]
- 73.Forlano R, Mullish BH, Giannakeas N, et al. High-throughput, machine learning-based quantification of steatosis, inflammation, ballooning, and fibrosis in biopsies from patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;18(9): 2081–90.e9. 10.1016/j.cgh.2019.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Vincent R, Yang J, et al. Dual-photon microscopy-based quantitation of fibrosis-related parameters (q-FP) to model disease progression in steatohepatitis. Hepatology. 2017;65(6):1891–1903. 10.1002/hep.29090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhaegh P, Bavalia R, Winkens B, Masclee A, Jonkers D, Koek G. Noninvasive tests do not accurately differentiate nonalcoholic steatohepatitis from simple steatosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(6):837–61. 10.1016/j.cgh.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 76.Newsome PN, Sasso M, Deeks JJ, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(4):362–73. 10.1016/S2468-1253(19)30383-8. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boursier J, Anty R, Vonghia L, et al. Screening for therapeutic trials and treatment indication in clinical practice: MACK-3, a new blood test for the diagnosis of fibrotic NASH. Aliment Pharmacol Ther. 2018;47(10):1387–96. 10.1111/apt.14621. [DOI] [PubMed] [Google Scholar]
- 78.Harrison SA, Ratziu V, Boursier J, et al. A blood-based biomarker panel (NIS4) for non-invasive diagnosis of non-alcoholic steatohepatitis and liver fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5(10): 970–85. 10.1016/S2468-1253(20)30252-1. [DOI] [PubMed] [Google Scholar]
- 79.Glen J, Floros L, Day C, Pryke R; Guideline Development Group. Non-alcoholic fatty liver disease (NAFLD): summary of NICE guidance. BMJ. 2016;354:i4428. 10.1136/bmj.i4428. Medline: [DOI] [PubMed] [Google Scholar]
- 80.Vali Y, Lee J, Boursier J, et al. Enhanced liver fibrosis test for the non-invasive diagnosis of fibrosis in patients with NAFLD: a systematic review and meta-analysis. J Hepatol. 2020; 73(2):252–62. 10.1016/j.jhep.2020.03.036. [DOI] [PubMed] [Google Scholar]
- 81.Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70(5):1521–30. 10.1002/hep.30842. Medline: [DOI] [PubMed] [Google Scholar]
- 82.Boursier JS, Sanyal AJ, Ratziu V, et al. Noninvasive assessments to identify patients with advanced fibrosis due to NASH: screened population from the REGENERATE trial. [Oral abstract]. Hepatology. 2020;72(S1):Section 56. 10.1002/hep.31578. [DOI] [Google Scholar]
- 83.Kosick HM, Keyrouz A, Adeyi O, Sebastiani G, Patel K. A stepwise algorithmic approach and external validation study for noninvasive prediction of advanced fibrosis in nonalcoholic fatty liver disease. Dig Dis Sci. 2021. Jan 3. Epub ahead of print. 10.1007/s10620-020-06748-8. Medline: [DOI] [PubMed] [Google Scholar]
- 84.Srivastava A, Gailer R, Tanwar S, et al. Prospective evaluation of a primary care referral pathway for patients with non-alcoholic fatty liver disease. J Hepato 2019; 71(2):371–8. 10.1016/j.jhep.2019.03.033. [DOI] [PubMed] [Google Scholar]
- 85.Lee J, Vali Y, Boursier J, et al. Prognostic accuracy of FIB-4, NAFLD fibrosis score and APRI for NAFLD-related events: a systematic review. Liver Int. 2021;41(2):261–70. 10.1111/liv.14669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sanyal AJ, Amstee QM, Trauner MH, et al. Cirrhosis regression is associated with improved clinical outcomes in patients with nonalcoholic steatohepatitis (NASH). Presented at The Liver Meeting: Digital Experience; 2020 Nov 11–16; Montreal, Quebec, Canada [virtual]. Hepatology. 2020; 72(S1):Article 90. Available from: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–30. 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 88.Shaheen AA, Riazi K, Medellin A, et al. Risk stratification of patients with nonalcoholic fatty liver disease using a case identification pathway in primary care: a cross-sectional study. CMAJ Open. 2020;8(2):E370–6. 10.9778/cmajo.20200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karlas T, Petroff D, Sasso M, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2016;66(5): 1022–30. 10.1016/j.jhep.2016.12.022. Medline: [DOI] [PubMed] [Google Scholar]
- 90.Yokoo T, Serai SD, Pirasteh A, et al. Linearity, bias, and precision of hepatic proton density fat fraction measurements by using MR imaging: a meta-analysis. Radiology. 2018;286(2):486–98. 10.1148/radiol.2017170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang A, Desai A, Hamilton G, et al. Accuracy of MR imaging-estimated proton density fat fraction for classification of dichotomized histologic steatosis grades in nonalcoholic fatty liver disease. Radiology. 2015;274(2):416–25. 10.1148/radiol.14140754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamilton G, Schlein AN, Wolfson T, et al. The relationship between liver triglyceride composition and proton density fat fraction as assessed by 1H MRS. NMR Biomed. 2020;33(6):e4286. 10.1002/nbm.4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mariappan YK, Glaser KJ, Ehman RL. Magnetic resonance elastography: a review. Clin Anat. 2010;23(5):497–511. 10.1002/ca.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Imajo K, Honda Y, Kobayashi T, et al. Direct comparison of US and MR elastography for staging liver fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2020;S1542-3565(20): 31673-6. Epub 2020 Dec 16. 10.1016/j.cgh.2020.12.016. Medline: [DOI] [PubMed] [Google Scholar]
- 95.Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–40. 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between hepatic inflammation and fibrosis with MR elastography. Radiology. 2017;284(3):694–705. 10.1148/radiol.2017160622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McDonald N, Eddowes PJ, Hodson J, et al. Multiparametric magnetic resonance imaging for quantitation of liver disease: a two-centre cross-sectional observational study. Sci Rep. 2018;8: Article number 9189. 10.1038/s41598-018-27560-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lefebvre T, Hebert M, Bilodeau L, et al. Intravoxel incoherent motion diffusion-weighted MRI for the characterization of inflammation in chronic liver disease. Eur Radiol. 2021;31:1347–58. 10.1007/s00330-020-07203-y. [DOI] [PubMed] [Google Scholar]
- 99.Bischoff SC, Bernal W, Dasarathy S, et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39(12):3533–62. 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 100.Kondrup J, Rasmussen HH, Hamberg O, Stanga Z; Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22(3):321–36. 10.1016/S0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 101.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–75. 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 102.Romero-Gomez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67(4): 829–46. 10.1016/j.jhep.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 103.Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–91. 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wycherley TP, Moran LJ, Clifton PM, Noakes M, Brinkworth GD. Effects of energy-restricted high-protein, low-fat compared with standard-protein, low-fat diets: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2012;96(6):1281–98. 10.3945/ajcn.112.044321. [DOI] [PubMed] [Google Scholar]
- 105.Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med. 2010;363:2102–13. 10.1056/NEJMoa1007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sabag A, Way KL, Keating SE, et al. Exercise and ectopic fat in type 2 diabetes: a systematic review and meta-analysis. Diabetes Metab. 2017;43(3):195–210. 10.1016/j.diabet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 107.Mann S, Jimenez A, Steele J, Domone S, Wade M, Beedie C. Programming and supervision of resistance training leads to positive effects on strength and body composition: results from two randomised trials of community fitness programmes. BMC Public Health. 2018;18 :Article number 420. 10.1186/s12889-018-5289-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Vos MB, Lavine JE. Dietary fructose in nonalcoholic fatty liver disease. Hepatology. 2013;57(6):2525–31. 10.1002/hep.26299. [DOI] [PubMed] [Google Scholar]
- 109.Lazarus JV, Ekstedt M, Marchesini G, et al. A cross-sectional study of the public health response to non-alcoholic fatty liver disease in Europe. J Hepatol. 2020;72(1):14–24. 10.1016/j.jhep.2019.08.027. [DOI] [PubMed] [Google Scholar]
- 110.Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78. 10.1053/j.gastro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 111.Gepner Y, Shelef I, Komy O, et al. The beneficial effects of Mediterranean diet over low-fat diet may be mediated by decreasing hepatic fat content. J Hepatol. 2019;71(2):379–88. 10.1016/j.jhep.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 112.Yaskolka Meir A, Rinott E, Tsaban G, et al. Effect of green-Mediterranean diet on intrahepatic fat: the DIRECT PLUS randomised controlled trial. Gut. 2021; gutjnl-2020-323106. Epub 2021 Jan 18. 10.1136/gutjnl-2020-323106. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scragg J, Avery L, Cassidy S, et al. Feasibility of a very low calorie diet to achieve a sustainable 10% weight loss in patients with nonalcoholic fatty liver disease. Clin Transl Gastroenterol. 2020;11(9):e00231. 10.14309/ctg.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lassailly G, Caiazzo R, Buob D, et al. Bariatric surgery reduces features of nonalcoholic steatohepatitis in morbidly obese patients. Gastroenterology. 2015;149:(2)379–88. 10.1053/j.gastro.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 115.Hashida R, Kawaguchi T, Bekki M, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J Hepatol. 2017;66(1):142–52. 10.1016/j.jhep.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 116.Musso G, Gambino R, Cassader M, Pagano G. A meta-analysis of randomized trials for the treatment of nonalcoholic fatty liver disease. Hepatology. 2010;52(1):79–104. 10.1002/hep.23623. [DOI] [PubMed] [Google Scholar]
- 117.Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut. 2013;62:606–15. 10.1136/gutjnl-2011-301708. [DOI] [PubMed] [Google Scholar]
- 118.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet. 2016;387(10019):679–90. 10.1016/S0140-6736(15)00803-X. [DOI] [PubMed] [Google Scholar]
- 119.Newsome PN, Buchholtz K, Cusi K, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384:1113–24. 10.1056/NEJMoa2028395. [DOI] [PubMed] [Google Scholar]
- 120.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cusi K, Orsak B, Bril F, et al. Long-term pioglitazone treatment for patients with nonalcoholic steatohepatitis and prediabetes or type 2 diabetes mellitus: a randomized trial. Ann Intern Med. 2016;165:305–15. 10.7326/M15-1774. [DOI] [PubMed] [Google Scholar]
- 122.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet. 2019;394(10215):2184–96. 10.1016/S0140-6736(19)33041-7. [DOI] [PubMed] [Google Scholar]
- 123.Loomba R, Sanyal AJ, Kowdley KV, et al. Factors associated with histologic response in adult patients with nonalcoholic steatohepatitis. Gastroenterology. 2019;156(1):88–95.e5. 10.1053/j.gastro.2018.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Patel K, Harrison SA, Elkhashab M, et al. Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: a phase 2 randomized controlled trial. Hepatology. 2020;72(1):58–71. 10.1002/hep.31205. [DOI] [PubMed] [Google Scholar]
- 125.Harrison SA, Neff G, Guy CD, et al. Efficacy and safety of aldafermin, an engineered FGF19 analog, in a randomized, double-blind, placebo-controlled trial of patients with nonalcoholic steatohepatitis. Gastroenterology. 2021;160(1):219–31.e1. 10.1053/j.gastro.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 126.Lefere S, Puengel T, Hundertmark J, et al. Differential effects of selective-and pan-PPAR agonists on experimental steatohepatitis and hepatic macrophages. J Hepatol. 2020; 73(4):757–70. 10.1016/j.jhep.2020.04.025. [DOI] [PubMed] [Google Scholar]
- 127.Frias JP, Nauck MA, Van J, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–93. 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 128.Safadi R, Konikoff FM, Mahamid M, et al. The fatty acid-bile acid conjugate aramchol reduces liver fat content in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12(12):2085–91.e1. 10.1016/j.cgh.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 129.Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet. 2019;394(10213):2012–24. 10.1016/S0140-6736(19)32517-6. [DOI] [PubMed] [Google Scholar]
- 130.Tilg H, Effenberger M. From NAFLD to MAFLD: when pathophysiology succeeds. Nat Rev Gastroenterol Hepatol. 2020;17:387–8. 10.1038/s41575-020-0316-6. [DOI] [PubMed] [Google Scholar]
- 131.Barrera F, George J. The role of diet and nutritional intervention for the management of patients with NAFLD. Clin Liver Dis. 2014;18(1):91–112. 10.1016/j.cld.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 132.Taylor R, Barnes AC. Translating aetiological insight into sustainable management of type 2 diabetes. Diabetologia. 2018;61:273–83. 10.1007/s00125-017-4504-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Loomba R, Abraham M, Unalp A, et al. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology. 2012;56(3):943–51. 10.1002/hep.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gastaldelli A, Cusi K. From NASH to diabetes and from diabetes to NASH: mechanisms and treatment options. JHEP Rep. 2019;1(4): 312–28. 10.1016/j.jhepr.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40(3):419–30. 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 136.Bajaj HS, Brown RE, Bhullar L, Sohi N, Kalra S, Aronson R. SGLT2 inhibitors and incretin agents: associations with alanine aminotransferase activity in type 2 diabetes. Diabetes Metab. 2018;44(6):493–9. 10.1016/j.diabet.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 137.Choi DH, Lee SJ, Kang CD, et al. Nonalcoholic fatty liver disease is associated with coronary artery disease in Koreans. World J Gastroenterol. 2013;19(38):6453–7. 10.3748/wjg.v19.i38.6453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhao YC, Zhao GJ, Chen Z, She ZG, Cai J, Li H. Nonalcoholic fatty liver disease: an emerging driver of hypertension. Hypertension. 2020;75(2):275–84. 10.1161/HYPERTENSIONAHA.119.13419. [DOI] [PubMed] [Google Scholar]
- 139.Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M. Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care. 2007;30(11):2940–4. 10.2337/dc07-0792. [DOI] [PubMed] [Google Scholar]
- 140.Sun DQ, Wu SJ, Liu WY, et al. Association of low-density lipoprotein cholesterol within the normal range and NAFLD in the non-obese Chinese population: a cross-sectional and longitudinal study. BMJ Open. 2016;6:e013781. 10.1136/bmjopen-2016-013781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pisto P, Santaniemi M, Bloigu R, Ukkola O, Kesaniemi YA. Fatty liver predicts the risk for cardiovascular events in middle-aged population: a population-based cohort study. BMJ Open. 2014;4:e004973. 10.1136/bmjopen-2014-004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Baratta F, Pastori D, Angelico F, et al. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2020;18(10):2324–31.e4. 10.1016/j.cgh.2019.12.026. [DOI] [PubMed] [Google Scholar]
- 143.Karajamaki AJ, Patsi OP, Savolainen M, Kesaniemi YA, Huikuri H, Ukkola O. Non-alcoholic fatty liver disease as a predictor of atrial fibrillation in middle-aged population (OPERA Study). PLoS One. 2015;10:e0142937. 10.1371/journal.pone.0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lee JE, Lee YJ, Chung SY, Cho HW, Park BJ, Jung DH. Severity of nonalcoholic fatty liver disease is associated with subclinical cerebro-cardiovascular atherosclerosis risk in Korean men. PLoS One. 2018;13:e0193191. 10.1371/journal.pone.0193191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Soto-Angona O, Anmella G, Valdes-Florido MJ, et al. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18: Article number 261 10.1186/s12916-020-01713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]


