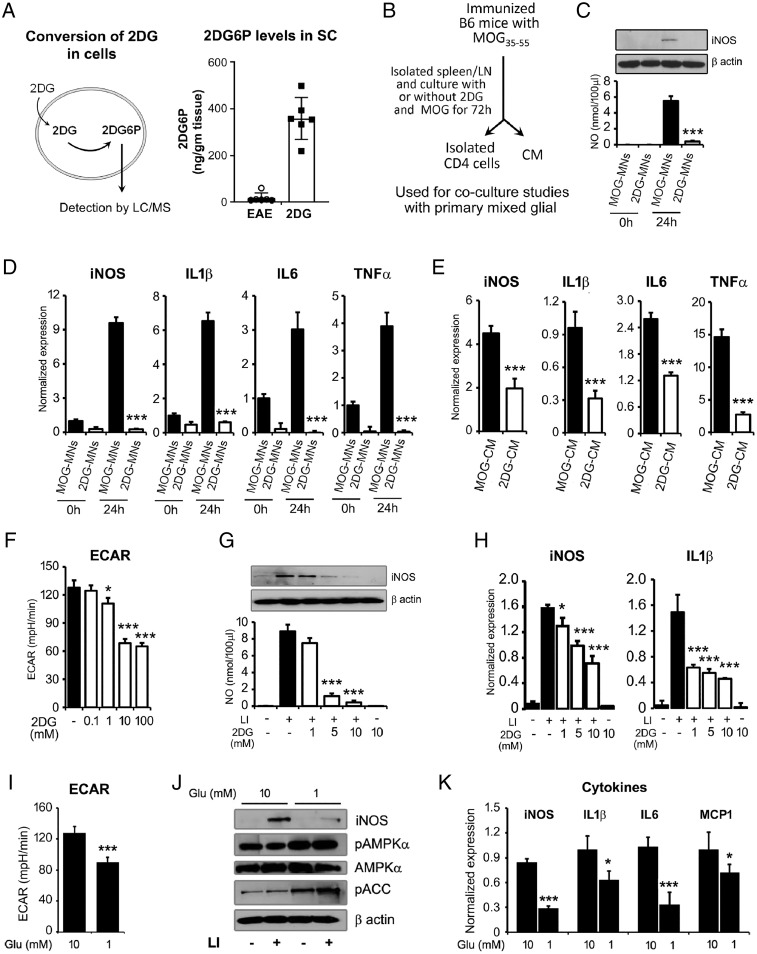
Fig. 7.
Reduction in glycolysis inhibits activation of brain glial cells. (A) 2-deoxyglucose (2DG) is taken up by cells/tissues and phosphorylated to produce 2-deoxyglucose-6-phosphate (2DG6P), which cannot be metabolized further. Accumulated levels of 2DG6P was detected by LC/MS in the spinal cord of EAE and 2DG-treated EAE. Data were shown as mean ± SD (n = 6 mice per group). (B) Schematic flow of experimental design to generate CM and activated MNs cells in the presence or absence of 2DG (1 mM) and used for further experiments. (C, D) For in vitro coculture model, treated and untreated MOG-primed MNs cells with 2DG were cocultured with primary brain glial cells (4:1 ratios of MNs cells:mixed glial cells) for 24h and were processed for nitric oxide (NO), iNOS, and cytokines expression by immunoblot and qPCR. The data presented are the mean ± SD of three values per group. (E) MOG-CM and 2DG-CM were added in culture media of mixed glia (1:1 ratio with serum-free RPMI) and expression of inflammatory mediator (iNOS) and cytokines (IL-1β, IL-6, and TNF-α) was examined by qPCR. (F) Dose-dependent effect of 2DG (0.1 to 100 mM) on ECAR in brain glial cells using Seahorse Bioanalyzer (n = 6 per group). (G, H) Brain glial cells were treated with 2DG (1 to 10 mM) for 30 min prior the treatment of LI (0.5 µg/10 ng per milliliter). Post 18 h, cells were processed for iNOS immunoblot analysis and cell supernatant was processed for NO. Under similar experimental condition, at 6 h of LI stimulation, cells were processed for RNA isolation and qPCR for the detection of iNOS and IL-1β. The data presented are the mean ± SD of three values per group. (I) Impact of glucose on ECAR in brain glial cells using Seahorse Bioanalyzer (n = 6 per group). (J) Primary brain glial cells were cultured in normal (10 mM) and low (1 mM) glucose condition in RPMI for 24 h followed by stimulation with LI for 18 h. The levels of iNOS, pAMPKα, AMPKα, and pACC were examined by immunoblot analysis using their specific antibodies. Beta actin was used to examine the equal protein loading. Represented blots are the one of the two independent experiments. (K) Primary brain glial cells were cultured in normal (10 mM) and low (1 mM) glucose condition in RPMI for 24 h followed by stimulation with LI for 6 h. Cells were processed for RNA isolation and qPCR for the detection of iNOS, IL-1β, IL6, and MCP1. The data presented are the mean ± SD of three values per group. *P < 0.05, **P < 0.01, and ***P < 0.001. Student t test, one-way analysis of variance.