Significance
As the world’s population of older adults increases, understanding disparities in age-related health is essential. Age-related changes in the immune system play a critical role in age-related morbidity and mortality. This study assesses associations between social stress and immunophenotypes as immune age phenotype markers for the first time in a national sample of older US adults. This study helps clarify mechanisms involved in accelerated development of the immune age phenotype, including socioeconomic and lifestyle factors and cytomegalovirus infection and reactivation. This study also identifies important points of intervention that may be useful in addressing inequalities in aging.
Keywords: socioeconomic status, immunosenescence, aging
Abstract
Exposure to stress is a risk factor for poor health and accelerated aging. Immune aging, including declines in naïve and increases in terminally differentiated T cells, plays a role in immune health and tissue specific aging, and may contribute to elevated risk for poor health among those who experience high psychosocial stress. Past data have been limited in estimating the contribution of life stress to the development of accelerated immune aging and investigating mediators such as lifestyle and cytomegalovirus (CMV) infection. This study utilizes a national sample of 5,744 US adults over age 50 to assess the relationship of social stress (viz., everyday discrimination, stressful life events, lifetime discrimination, life trauma, and chronic stress) with flow cytometric estimates of immune aging, including naïve and terminally differentiated T cell percentages and the ratio of CD4+ to CD8+ cells. Experiencing life trauma and chronic stress was related to a lower percentage of CD4+ naïve cells. Discrimination and chronic stress were each associated with a greater percentage of terminally differentiated CD4+ cells. Stressful life events, high lifetime discrimination, and life trauma were related to a lower percentage of CD8+ naïve cells. Stressful life events, high lifetime discrimination, and chronic stress were associated with a higher percentage of terminally differentiated CD8+ cells. High lifetime discrimination and chronic stress were related to a lower CD4+:CD8+ ratio. Lifestyle factors and CMV seropositivity partially reduced these effects. Results identify psychosocial stress as a contributor to accelerating immune aging by decreasing naïve and increasing terminally differentiated T cells.
Lifetime exposure to stressful conditions is a known risk factor for poorer health, increasing the risk for early onset of age-related disease and premature death (1–3). Models examining the mechanisms driving these effects have centered on the sequelae of repeated activation and prolonged activation of the sympathoadrenal and hypothalamic pituitary adrenal systems, leading to wear and tear at the biological level (4). Many have postulated that this wear and tear manifests at the cellular level, causing accumulation of DNA damage, increasing inflammation, shortening telomere length, and driving cellular aging (5, 6). Critically short telomere length within immune cells and cellular stress (e.g., DNA damage) can drive cells into a nonreplicating state termed cellular senescence (7, 8). In addition to localized tissue-specific aging (9), age-related changes in immune function contribute to systemic aging, organ failure, and premature mortality, making immunosenescence a critical player in aging and disease (10).
Age is a robust determinant of immune cell population composition (11, 12), with aging immune systems characterized by a reduced pool of naïve B and T cells, increased pool of terminally differentiated T cells, increased supply of CD8+ cells relative to CD4+ T cells, and increased systemic inflammation (13). However, there is substantial variance in the rate of these changes and consequent alterations in immune cell composition among older adults (14). Understanding the contributors to this variance is critical given that immune aging is associated with chronic diseases [e.g., cancer (15) and cardiovascular disease (16)], weakened response to acute infections, increased risk of pneumonia, reduced efficacy of vaccines (17), and organ system aging (10).
In the current study we focus on social stressors, that is, difficult or challenging circumstances (18) that arise from social position and experience that are expected to be stressful, that occur in adulthood. Past research has categorized stressors in various ways, often depending on discipline; however, stressors are typically distinguished by timescale (e.g., acute, daily hassles, life events, chronic stressors) (19). We distinguish discrimination-related stressors from other stressors because past research suggests these stressors have distinct, independent effects on health (20–22). We also distinguish traumatic life events from other stressors, as is common in past research (19, 23, 24). Thus, we assess five stress variables that have been well established past research and are available in a large number of studies of health and aging (viz., stressful life events, chronic stress, everyday discrimination, lifetime discrimination, and life trauma). More information about these stressors is available in the SI Appendix.
Social stress can modify the immune system in several ways, including increased inflammatory signaling and reduced antiviral responses (18, 25–27), suggesting that social stress may accelerate immune aging (28–38). However, past research has utilized small clinical and specialized samples, potentially limiting power to detect effects, power to assess mediators, and generalizability of results. In the present study, we utilized a nationwide sample of 5,641 US adults over the age of 50 to assess associations between five categories of social stress that have well established effects on heath and enumerative measures of immunological aging, including naïve CD4+ T cell percentages (CD4+/CD3+/CD19−/CD45RA+/CCR7+/CD28+) naïve CD8+ T cell percentages (CD8+/CD3+/CD19−/CD45RA+/CCR7+/CD28+), terminally differentiated effector memory CD4+ T cell percentages (TemRA; CD4+/CD3+/CD19−/CD45RA+/CCR7−/CD28−), terminally differentiated effector memory CD8+ T cell percentages (CD8+/CD3+/CD19−/CD45RA+/CCR7−/CD28−), and the ratio of CD4+ to CD8+ T cells. Analyses also assessed potential mediation of these associations by socioeconomic and lifestyle factors and cytomegalovirus (CMV) seropositivity.
Results
Descriptive statistics are shown in SI Appendix, Table S1. The weighted sample is 55.2% female and has a median age of 68 y. Of the sample, 84.9% is non-Hispanic White, 6.9% is non-Hispanic Black, 5.8% is Hispanic, and 2.5% is non-Hispanic other race; 10.1% has less than 12 y of education, 30.7% has 12 y of education, 26.1% has 13 to 15 y of education, and 33.1% has 16 or more years of education. Of the sample, 9.4% are current smokers and 45.4% are former smokers, more than one-third of the sample is obese (36.1%), and 53.6% were nondrinkers. On average, participants reported relatively low everyday discrimination, 0.50 stressful life events, 0.65 incidents of lifetime discrimination, 1.09 life traumas, and a moderate level of chronic stress. There was substantial variance on all of these scales.
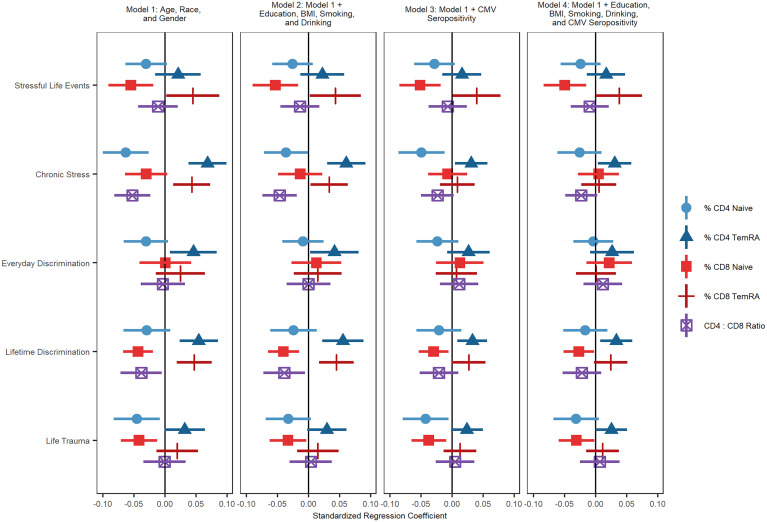
Results from nested analyses regressing T cell subset percentage/ratio on each stressor while controlling for age, sex, race/ethnicity, and various potential mediators (education, drinking, smoking, body mass index [BMI], and CMV serostatus), are shown in Fig. 1. Individual figures and tables for each predictor are shown in SI Appendix, Figs. S1–S5 and Tables S2–S6. All stress variables and immune outcomes variables were standardized to have a mean of 0 and SD of 1.
Fig. 1.
Regression coefficients and 95% CIs from nested analyses regressing cell subset percentage/ratio on each stressor and mediators. All models control for age, race, and sex.
Experiencing more stressful life events was associated with a lower percentage of CD8+ naïve T cells (b = −0.055, SE = 0.018, P = 0.004) and a higher percentage of terminally differentiated CD8+ T cells (b = 0.045, SE = 0.022, P = 0.044). The association between terminally differentiated CD8+ T cells and more stressful life events was reduced to nonsignificance after controlling for education, BMI, smoking, drinking and CMV seropositivity. The association between stressful life events and CD8+ naïve T cell percentage remained statistically significant after controlling for all covariates (b = −0.049, SE = 0.017, P = 0.007).
Experiencing greater chronic stress was related to a greater percentage of terminally differentiated CD4+ (b = 0.068, SE = 0.016, P < 0.001) and terminally differentiated CD8+ T cells (b = 0.043, SE = 0.015, P = 0.007), a lower percentage of CD4+ naïve T cells (b = −0.063, SE = 0.019, P = 0.001), and a lower ratio of CD4+ to CD8+ T cells (b = −0.053, SE = 0.015, P = 0.001). The association between chronic stress and CD4+ naïve T cells became nonsignificant after controlling for socioeconomic and lifestyle factors and CMV seropositivity. The associations between chronic stress and terminally differentiated CD8+ T cells and the CD4:CD8 ratio were reduced to nonsignificance after controlling for CMV seropositivity. The association between chronic stress and terminally differentiated CD4+ T cells (b = 0.031, SE = 0.014, P = 0.030) remained significant even after adjusting for education, BMI, smoking, drinking, and CMV seropositivity.
Greater lifetime discrimination was related to having higher percentages of terminally differentiated CD4+ (b = 0.054, SE = 0.016, P = 0.001) and terminally differentiated CD8+ T cells (b = 0.047, SE = 0.014, P = 0.002), a lower percentage of CD8+ naïve T cells (b = −0.043, SE = 0.012, P = 0.001), and a lower CD4+:CD8+ ratio (b = −0.038, SE = 0.017, P = 0.028). The association between terminally differentiated CD8+ T cells and greater lifetime discrimination was reduced to nonsignificance after controlling for education, BMI, smoking, drinking, and CMV seropositivity. The association between lifetime discrimination and the CD4+:CD4+ ratio was reduced to nonsignificance after controlling for CMV seropositivity. The associations between lifetime discrimination and terminally differentiated CD4+ (b = 0.033, SE = 0.013, P = 0.015) and CD8+ naïve (b = −0.027, SE = 0.013, P = 0.039) T cell percentages were both substantially reduced by these mediators but remained statistically significant.
Experiencing more everyday discrimination was related to having a higher percentage of terminally differentiated effector memory CD4+ T cells (b = 0.046, SE = 0.019, P = 0.022). This association was reduced after accounting for CMV seropositivity.
Finally, experiencing a greater number of life traumas was related to a smaller percentage of CD4+ naïve T cells (b = −0.045, SE = 0.019, P = 0.020) and CD8+ naïve T cells (b = −0.042, SE = 0.015, P = 0.007). The association between CD4+ naïve T cells and trauma was reduced to nonsignificance after controlling for education, BMI, smoking, and drinking, suggesting that these socioeconomic and lifestyle factors may mediate this association. Associations between life trauma and CD8+ naïve T cells (b = −0.031, SE = 0.015, P = 0.042) remained significant after including all covariates.
We also estimated models with interactions between stressors and sex and race/ethnicity, including all controls. There was only one statistically significant interaction. The effect of experiencing more lifetime discrimination on naïve CD8+ T cell percentage was amplified for Hispanic respondents compared to non-Hispanic White respondents.
Discussion
Using a national sample of older US adults, we found that exposure to social stress was associated with T cell distributions indicative of accelerated immune aging. Specifically, life trauma and chronic stress were associated with a lower percentage of CD4+ naïve T cells, whereas everyday discrimination, lifetime discrimination, and chronic stress were associated with a greater percentage of terminally differentiated CD4+ T cells. Stressful life events, lifetime discrimination and life trauma were associated with a lower percentage of CD8+ naïve T cells, whereas stressful life events, lifetime discrimination, and chronic stress were significantly associated with a higher percentage of terminally differentiated CD8+ T cells. Lifetime discrimination and chronic stress was associated with a lower CD4:CD8 ratio. These effects were all independent of chronological age, sex, and race/ethnicity.
All significant associations with CD4+ naïve T cells were reduced to nonsignificance after controlling for socioeconomic and lifestyle factors (viz., education, smoking, BMI, and alcohol use) and CMV seropositivity, suggesting that this may be an important pathway by which stress affects CD4+ T cell immunosenescence. This is consistent with past research suggesting that adiposity is associated with thymic involution (39) and research showing reduced percentages of CD4+ naïve T cells in people with alcohol use disorder (40). Further research is needed to clarify how these factors affect naïve CD4+ T cells.
Lifetime discrimination and chronic stress associations with terminally differentiated CD8+ T cells and the CD4:CD8 ratio became nonsignificant after controlling for CMV seropositivity and the association between stressful life events and terminally differentiated CD8+ T cells became nonsignificant after controlling for lifestyle and socioeconomic status factors and CMV seropositivity. This is consistent with past research showing that stress is associated with impaired immunological control of latent viruses like CMV (26). More stressed individuals may have reduced control over and more frequent activation of CMV, leading to an increase in memory T cells and decrease in the CD4:CD8 ratio (18, 41–43).
The percentage of CD8+ naïve T cells remained significantly inversely associated with stressful life events, lifetime discrimination, and life trauma regardless of controls added to the model. This suggests that the accumulation of major negative events across the life course may have a direct effect on these naïve CD8+ T cells, although future research may uncover mediating mechanisms that were not identified here. This finding is again consistent with the possibility that thymic involution may be accelerated by social stress [e.g., via chronic activation of the hypothalamic-pituitary-adrenal axis, which can accelerate thymic involution and thereby reducing naïve T cell development (25, 44)].
Finally, lifetime discrimination and chronic stress are associated with a greater percentage of terminally differentiated CD4+ T cells, regardless of controls in the model. CD4+ T cells play a critical role in cell-mediated immunity, producing cytokines that help direct the action of other cells. This compartment typically develops age-related changes later than the CD8+ compartment (17); therefore, accelerated immune age phenotype in CD4+ T cells may be particularly indicative of aging immunity. Research has shown these terminally differentiated CD4+ T cells fail to optimally “help” B cell activation, produce more inflammatory cytokines, and have been implicated in cardiovascular disease (16). This cell subset may be of particular interest to researcher investigating stress and health (45).
The current study has several limitations. This is a study of community-dwelling older adults in the United States. Future studies using non-US samples are needed. Our sample represents a cohort of older adults exposed to various forms of stress, discrimination, and trauma that might not be representative of younger cohorts or groups not well represented in the US Health and Retirement Survey (e.g., indigenous peoples). The most recent reports of life events (viz., stressful life events, lifetime discrimination, and life trauma) were in 2012. Thus, these measures may miss events that took place between 2012 and 2016. Respondents with particularly high exposure to stress may have died before reaching older ages, potentially attenuating the effect of stress on immune function. Because immunotyping data are only available at one time point, it is impossible to establish temporal ordering of stress and immune variables. We are not characterizing age-related changes across all cell types in the immune system. We selected T cells because of their importance in immune aging (see supporting information). Future research is needed to characterize stress effects on age-related changes in other cell types (e.g., B cells, monocytes, natural killer cells). Additionally, we are not able to address all domains and timescales of stressors. We focus on five well-established and widely used stress scales; however, future research should investigate other domains of stress (e.g., job stress, caregiver stress).
Despite these limitations, this study provides important insights on the role of social stress in immune aging, highlighting a key role for health behaviors and social-environmental conditions as correlates of naïve T cell decline as well as a distinctive association of stressors with higher terminally differentiated CD4+ T cell percentages (i.e., independent of variables controlled for here). These results raise the possibility that interventions such as CMV vaccination and senolytic therapies might potentially help reduce social disparities in T cell immunologic aging (13). Interventions aimed at reducing stress or increasing resilience may be needed to address these inequalities.
Materials and Methods
Sample.
We utilize data from the US Health and Retirement Study (HRS) 2016 Venous Blood Study (n = 9,934). Multiparameter flow cytometry was used to assess counts and percentages of 24 different types of immune cells using the standardized protocol by the Human Immunology Project (46) with minor modifications performed on an LSRII or a Fortessa ×20 flow cytometer (BD Biosciences) More detailed methods for this sample are published elsewhere (47–49).
This sample was designed to be representative of the US population when weighted. Stress scales were assessed as part of the psychosocial leave behind questionnaire at various waves. This questionnaire is given to a rotating random half of the core panel every other wave so that data are available for the full sample every 4 y. Two hundred participants were in a nursing home or were cohort ineligible in 2016, 614 participants were missing on one or more cell type, 3,233 participants were missing on one or more stressor, and 142 participants were missing on one or more mediator or control variables. Thus, the final sample size is 5,744 (SI Appendix, Fig. S6).
Measures.
T lymphocyte distributions were assessed following the protocol described above. We focus here on the percentage of naïve CD4+ and CD8+ T cells (CD3+/CD19−/CD45RA+/CCR7+/CD28+) and TemRA CD4+ and CD8+ T cells (CD3+/CD19−/CD45RA+/CCR7−/CD28−). We also assess the ratio of CD4+ to CD8+ T cells using the quotient of counts of these compartments. Highly skewed cell variables (skewness > 2; viz., CD4+ TemRA and the CD4:CD8 ratio) were natural log transformed to approximate a normal distribution. These variables were standardized to have a mean of 0 and SD of 1 to ease comparison.
We utilize five well-established health-relevant measures of social stress: stressful life events (50), chronic stress (51), everyday discrimination (52), lifetime discrimination (52), and life trauma (53). Because everyday discrimination, stressful life events, lifetime discrimination, and life trauma assess life course or ongoing exposure to stress, we use the first available data from the years these measures were assessed (2006 to 2016 for everyday discrimination, 2008 to 2012 for stressful life events, and 2006 to 2012 for lifetime discrimination and life trauma). Because we are interested in assessing ongoing chronic stress, we only use data from the 2014 and 2016 leave behind questionnaire. More information on these measures is available in the SI Appendix and more detailed information is available elsewhere (54).
Socioeconomic and lifestyle factors were assessed using self-reports of education (categorized: 0 to 11 y, 12 y, 13 to 15 y, and 16+ years as the reference), self-reported smoking (categorized: current smoker, past smoker, and nonsmoker as the reference), BMI (categorized: ≥ 25 and < 30 overweight, ≥ 30 and < 35 obese 1, ≥ 35 obese 2, and < 25 normal and underweight as the reference), and alcohol use (categorized: 1 to 4 drinks per day drinking, 5+ drinks per day drinking, and nondrinker as the reference).
CMV seropositivity was assessed using IgG antibodies in serum with the Roche e411 immunoassay analyzer (Roche Diagnostics Corporation) (categorized: borderline, reactive, or nonreactive as the reference) (49).
In all models we control for chronological age, race/ethnicity (non-Hispanic Black, Hispanic, bon-Hispanic other race, and non-Hispanic White as reference), and sex (male as reference).
Plan of Analysis.
All stressors and T cell type percentages/ratios were standardized to have a mean of 0 and SD of 1 to ease comparisons across models. A series of nested linear regressions were estimated regressing each T cell type percentage/ratio first on controls, then on controls and socioeconomic and lifestyle factors, then on controls and CMV seropositivity, and finally on controls, socioeconomic and lifestyle factors and CMV seropositivity. All analyses were conducted in R 4.1.1 (55) using the survey package (56).
Supplementary Material
Acknowledgments
Support was provided by the USC/UCLA Center on Biodemography and Population Health through a grant from the National Institute on Aging (P30AG017265). The US Health and Retirement Study is sponsored by the National Institute on Aging (Grant Number NIA U01AG009740) and is conducted by the University of Michigan.
Footnotes
Reviewers: J.K.-G., Ohio State University; and I.S., The Pennsylvania State University.
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2202780119/-/DCSupplemental.
Data Availability
Anonymized data have been deposited in https://hrsdata.isr.umich.edu/. Study data are available (researchers must submit an application to https://hrs.isr.umich.edu/about).
References
- 1.Turner R. J., “Understanding health disparities: The promise of the stress process model” in Advances in the Conceptualization of the Stress Process, Avison W. R., Aneshensel C. S., Schieman S., Wheaton B., Eds. (Springer, 2009), pp. 3–21. [Google Scholar]
- 2.Kivimäki M., Steptoe A., Effects of stress on the development and progression of cardiovascular disease. Nat. Rev. Cardiol. 15, 215–229 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Cohen S., “Psychological stress, immunity, and physical disease” in Scientists Making a Difference: The Greatest Living Behavioral and Brain Scientists Talk about Their Most Important Contributions, Sternberg S., Fiske S., Foss D., Eds. (Cambridge University Press, 2016), pp. 419–423. [Google Scholar]
- 4.McEwen B. S., Seeman T., Protective and damaging effects of mediators of stress. Elaborating and testing the concepts of allostasis and allostatic load. Ann. N. Y. Acad. Sci. 896, 30–47 (1999). [DOI] [PubMed] [Google Scholar]
- 5.Epel E. S., et al. , Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 101, 17312–17315 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalev I., Hastings W. J., “Psychosocial stress and telomere regulation” in Genes, Brain, and Emotions: Interdisciplinary and Translational Perspectives, A. C. Miu, J. R. Homberg, K.-P. Lesch, Eds. (Oxford University Press, 2019), pp. 247–261. [Google Scholar]
- 7.Etzel L. C., Shalev I., “Effects of psychological stress on telomeres as genome regulators” in Stress: Genetics, Epigenetics and Genomics, G. Fink, Ed. (Elsevier, 2021), pp. 109–117. [Google Scholar]
- 8.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G., The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Childs B. G., Durik M., Baker D. J., van Deursen J. M., Cellular senescence in aging and age-related disease: From mechanisms to therapy. Nat. Med. 21, 1424–1435 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yousefzadeh M. J., et al. , An aged immune system drives senescence and ageing of solid organs. Nature 594, 100–105 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang Z., et al. , Effects of sex and aging on the immune cell landscape as assessed by single-cell transcriptomic analysis. Proc. Natl. Acad. Sci. U.S.A. 118, e2023216118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thyagarajan B., et al. , Age-related differences in T cell subsets in a nationally representative sample of people over age 55: Findings from the Health and Retirement Study. J. Gerontol. Ser. A 77, 927–933 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aiello A., et al. , Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 10, 2247 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaczorowski K. J., et al. , Continuous immunotypes describe human immune variation and predict diverse responses. Proc. Natl. Acad. Sci. U.S.A. 114, E6097–E6106 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huff W. X., Kwon J. H., Henriquez M., Fetcko K., Dey M., The evolving role of CD8+CD28- immunosenescent T cells in cancer immunology. Int. J. Mol. Sci. 20, 2810 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Broadley I., Pera A., Morrow G., Davies K. A., Kern F., Expansions of cytotoxic CD4+CD28- T cells drive excess cardiovascular mortality in rheumatoid arthritis and other chronic inflammatory conditions and are triggered by CMV infection. Front. Immunol. 8, 195 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pangrazzi L., Weinberger B., T cells, aging and senescence. Exp. Gerontol. 134, 110887 (2020). [DOI] [PubMed] [Google Scholar]
- 18.Reed R. G., Stress and immunological aging. Curr. Opin. Behav. Sci. 28, 38–43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Epel E. S., et al. , More than a feeling: A unified view of stress measurement for population science. Front. Neuroendocrinol. 49, 146–169 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geronimus A. T., et al. , Do US Black women experience stress-related accelerated biological aging? A novel theory and first population-based test of Black-White differences in telomere length. Hum. Nat. 21, 19–38 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geronimus A. T., Hicken M., Keene D., Bound J., “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am. J. Public Health 96, 826–833 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons R. L., et al. , Discrimination, segregation, and chronic inflammation: Testing the weathering explanation for the poor health of Black Americans. Dev. Psychol. 54, 1993–2006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine M., Traumatic life experiences are associated with increases in epigenetic aging. Biol. Psychiatry 83, S92–S93 (2018). [Google Scholar]
- 24.Levine M. E., Cole S. W., Weir D. R., Crimmins E. M., Childhood and later life stressors and increased inflammatory gene expression at older ages. Soc. Sci. Med. 130, 16–22 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaser R., Kiecolt-Glaser J. K., Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 5, 243–251 (2005). [DOI] [PubMed] [Google Scholar]
- 26.Gouin J.-P., Hantsoo L., Kiecolt-Glaser J. K., Immune dysregulation and chronic stress among older adults: A review. Neuroimmunomodulation 15, 251–259 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gouin J.-P., Glaser R., Malarkey W. B., Beversdorf D., Kiecolt-Glaser J., Chronic stress, daily stressors, and circulating inflammatory markers. Health Psychol. 31, 264–268 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bauer M. E., Chronic stress and immunosenescence: A review. Neuroimmunomodulation 15, 241–250 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Sommershof A., et al. , Substantial reduction of naïve and regulatory T cells following traumatic stress. Brain Behav. Immun. 23, 1117–1124 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Maes M., et al. , The effects of psychological stress on leukocyte subset distribution in humans: Evidence of immune activation. Neuropsychobiology 39, 1–9 (1999). [DOI] [PubMed] [Google Scholar]
- 31.Elwenspoek M. M. C., et al. , T cell immunosenescence after early life adversity: Association with cytomegalovirus infection. Front. Immunol. 8, 1263 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bosch J. A., Fischer J. E., Fischer J. C., Psychologically adverse work conditions are associated with CD8+ T cell differentiation indicative of immunesenescence. Brain Behav. Immun. 23, 527–534 (2009). [DOI] [PubMed] [Google Scholar]
- 33.Aiello A. E., et al. , Income and markers of immunological cellular aging. Psychosom. Med. 78, 657–666 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aiello A. E., et al. , PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology 67, 133–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rentscher K. E., et al. , Chronic stress exposure and daily stress appraisals relate to biological aging marker p16INK4a. Psychoneuroendocrinology 102, 139–148 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fagundes C. P., Glaser R., Kiecolt-Glaser J. K., Stressful early life experiences and immune dysregulation across the lifespan. Brain Behav. Immun. 27, 8–12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiecolt-Glaser J. K., McGuire L., Robles T. F., Glaser R., Psychoneuroimmunology: Psychological influences on immune function and health. J. Consult. Clin. Psychol. 70, 537–547 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Kiecolt-Glaser J. K., Glaser R., Stress and immunity: Age enhances the risks. Curr. Dir. Psychol. Sci. 10, 18–21 (2001). [Google Scholar]
- 39.Kalathookunnel Antony A., Lian Z., Wu H., T cells in adipose tissue in aging. Front. Immunol. 9, 2945 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zuluaga P., et al. , Loss of naive T lymphocytes is associated with advanced liver fibrosis in alcohol use disorder. Drug Alcohol Depend. 213, 108046 (2020). [DOI] [PubMed] [Google Scholar]
- 41.Bektas A., Schurman S. H., Sen R., Ferrucci L., Human T cell immunosenescence and inflammation in aging. J. Leukoc. Biol. 102, 977–988 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bauer M. E., Fuente Mde. L., The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 158, 27–37 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Reed R. G., Greenberg R. N., Segerstrom S. C., Cytomegalovirus serostatus, inflammation, and antibody response to influenza vaccination in older adults: The moderating effect of beta blockade. Brain Behav. Immun. 61, 14–20 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bauer M. E., Stress, glucocorticoids and ageing of the immune system. Stress 8, 69–83 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Pearlin L. I., The sociological study of stress. J. Health Soc. Behav. 30, 241–256 (1989). [PubMed] [Google Scholar]
- 46.Maecker H. T., McCoy J. P., Nussenblatt R., Standardizing immunophenotyping for the Human Immunology Project. Nat. Rev. Immunol. 12, 191–200 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thyagarajan B., et al. , Effect of delayed cell processing and cryopreservation on immunophenotyping in multicenter population studies. J. Immunol. Methods 463, 61–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barcelo H., Faul J., Crimmins E., Thyagarajan B., A practical cryopreservation and staining protocol for immunophenotyping in population studies. Curr. Protoc. Cytom. 84, e35 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crimmins E. M., Faul J. D., Thyagarajan B., Weir D. R., Venous Blood Collection and Assay Protocol in the 2016 Health and Retirement Study 2016 Venous Blood Study (VBS, 2017). [Google Scholar]
- 50.Turner R. J., Wheaton B., Lloyd D. A., The epidemiology of social stress. Am. Sociol. Rev. 60, 104–125 (1995). [Google Scholar]
- 51.Troxel W. M., Matthews K. A., Bromberger J. T., Sutton-Tyrrell K., Chronic stress burden, discrimination, and subclinical carotid artery disease in African American and Caucasian women. Health Psychol. 22, 300–309 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Williams D. R., Yu Yan, Jackson J. S., Anderson N. B., Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J. Health Psychol. 2, 335–351 (1997). [DOI] [PubMed] [Google Scholar]
- 53.Krause N., Shaw B. A., Cairney J., A descriptive epidemiology of lifetime trauma and the physical health status of older adults. Psychol. Aging 19, 637–648 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Smith J., Ryan L. H., Fisher G. G., Sonnega A., Weir D. R., HRS Psychosocial and Lifestyle Questionnaire 2006–2016 (Survey Research Center, Institute for Social Research, University of Michigan, 2017). [Google Scholar]
- 55.R Core Team, R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021). [Google Scholar]
- 56.Lumley T., Analysis of complex survey samples. J. Stat. Softw. 9, 1–19 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Anonymized data have been deposited in https://hrsdata.isr.umich.edu/. Study data are available (researchers must submit an application to https://hrs.isr.umich.edu/about).