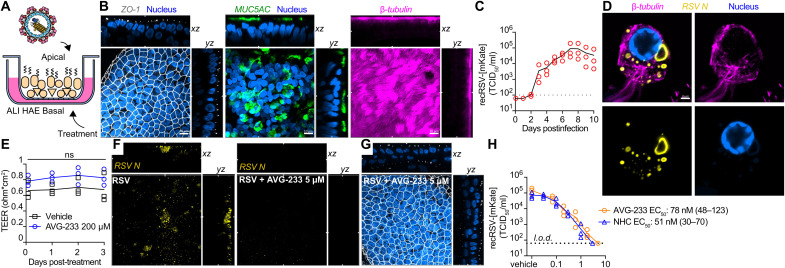
Fig. 4. Efficacy of AVG-233 in well-differentiated HAE (3D-HAE) cells grown at air-liquid interface.
(A) Schematics of 3D-HAE. (B) Confocal imaging of 3D-HAE. Tight junctions immunodetected with anti–ZO-I (white). Mucus-producing goblet cells immunodetected with anti-Muc5AC (green). Ciliated cells immunodetected with anti–β-tubulin (pink). Nuclei stained with Hoechst 35443 (blue). Scale bars, 10 μm. (C) Multistep growth curve of recRSV-mKate in 3D-HAEs. Viral titers were assayed from the apical chambers. Symbols show biological repeats (n = 3); curve connects means ± SD. (D) Immunolabeling of recRSV-fireSMASh–induced inclusion bodies with specific anti-RSV N antibody. Scale bar, 2 μm. (E) Transepithelial electrical resistance of 3D-HAEs exposed basolaterally to 200 μM AVG-233 or vehicle (DMSO) for up to 3 days. Symbols represent means ± SD (n = 3). Two-way ANOVA with Sidak post hoc test. (F) Immunostaining of RSV N in RSV-infected cells with or without AVG-233 5 μM at 3 days postinfection (d.p.i.). Scale as in (B). (G) Immunostaining of tight junctions in RSV-infected cells with AVG-233 5 μM at 3 d.p.i. Scale as in (B). (H) AVG-233 and NHC virus yield reduction against recRSV-mKate in 3D-HAEs. Progeny virus titers were determined 6 days after infection; symbols show biological repeats (n = 3); curve connects mean values. EC50 calculation through four-parameter variable slope regression modeling.