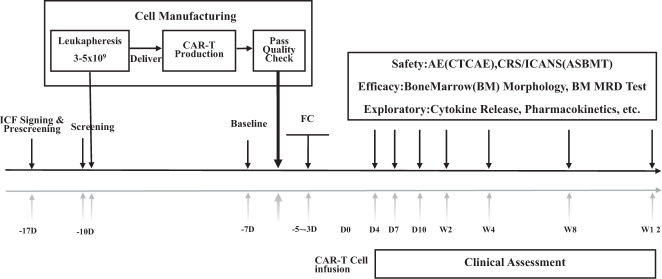
Fig. 1. Clinical study schema.
After screening the patients and signing informed consent form, the PBMCs were collected by leukapheresis. The GC007F cells were constructed and infused into patients after conditioning regimens. The pharmacokinetics, response assessment and adverse events were observed. ICF Informed consent form, PBMCs peripheral blood mononuclear cells, MRD minimal residual disease, AE advert effect, CRS cytokine release syndrome, ICANS immune effector cell-associated neurotoxicity syndrome, CTCAE common terminology criteria for adverse events, ASBMT American society for blood and marrow transplantation.