Fig. 4. AimP-induced conformation changes in AimR.
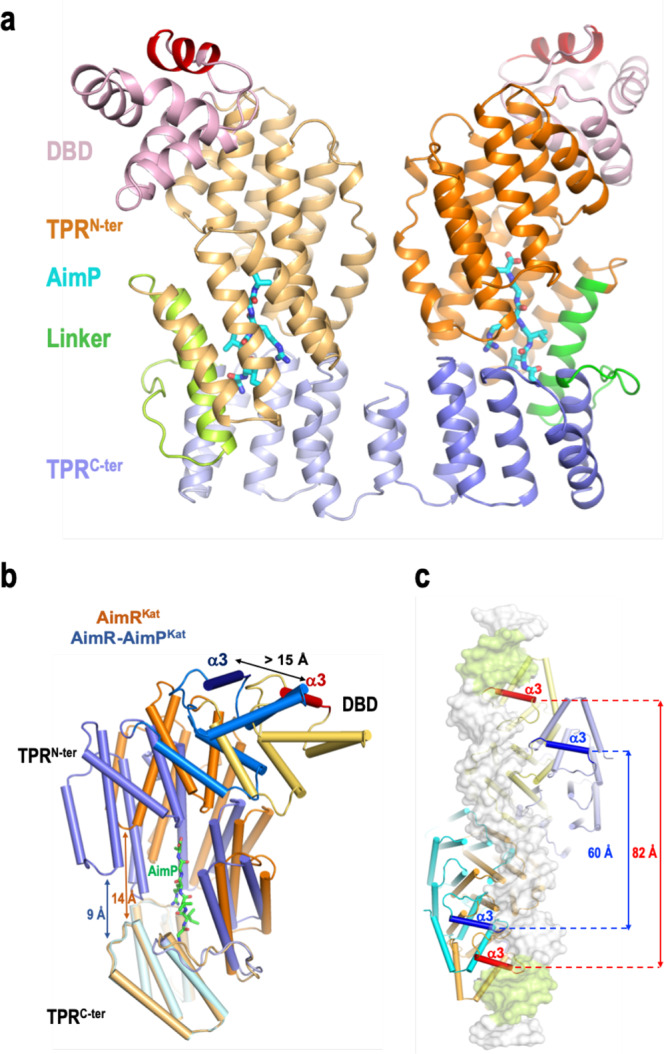
a Cartoon rendering of dimeric AimP-AimRKat complex. The structure is presented in similar view and colours as in Fig. 2b. AimPs are shown in sticks representation with carbon, oxygen and nitrogen atoms coloured in cyan, red and blue, respectively. b AimP induces a compact conformation of AimR. Superimposition of AimRKat protomers in its apo (orange colours) and AimP-bound (blue colours) states shows how the TPRN-ter (dark hue) and TPRc-ter (light hue) domains approaches as the peptide binds reducing their distance from 14 to 9 Å. This compaction movement promotes a displacement greater than 15 Å in the recognition helix α3. AimR are rendered with helices as cylinders and the AimP in sticks representation with carbon coloured in green. c AimP binding prevents DNA operator recognition. Superposition of AimRKat in its apo and AimP-bound conformations on the structure of AimRSPβ bound to its DNA operator (PDB 6HP7). A view from the DNA side with showing AimRKat structures in cartoon rendering as in (b) and the operator DNA on white semi-transparent surface with the palindromic sequences highlighted in green. The recognition helices are highlighted in a darker colour and intra-dimeric distance is indicated.
