Abstract
Objective
To examine factors associated with parent quality of life during and after NICU discharge among parents of infants with congenital anomalies admitted to the NICU.
Study design
Secondary analysis of two prospective cohort studies between 2016–2020 at a level IV NICU; parents of infants with major congenital anomalies receiving NICU care were enrolled. The primary outcomes were parent HRQL during the NICU stay and three-months post-NICU discharge
Results
166 parent-infant dyads were enrolled in the study, of which 124 completed the 3-month follow-up interview. During the NICU stay, parent history of a mental health disorder (−13 points), earlier gestational age (−17 points), consultation by multiple specialists (−11 points), and longer hospital stay (−5 points) were associated with lower HRQL. Parents of infants with a neonatal surgical anomaly had higher HRQL (+4 points). At 3 months after NICU discharge, parent receiving a psychology consult in the NICU, total number of consultants involved in the child’s care, and infants with non-surgical anomalies were associated with lower parent HRQL. Parents of infants with gastrostomy tubes (−6 points) and those who had hospital readmissions (−5 points) had lower HRQL. When we compared same-parent differences in HRQL over time, parents of infants with anomalies did not show significant improvement in HRQL upon discharge home.
Conclusion
Parents of infants with congenital anomalies reported low HRQL at baseline and at discharge. Parents of infants with non-surgical, medically complex anomalies requiring multispecialty care represent a vulnerable group who could be better supported during and after the NICU.
Keywords: quality of life, NICU, anomalies, surgical anomalies
Infants with congenital anomalies represent up to a third of children’s hospital neonatal intensive care unit (NICU) admissions.(1) Parents of infants with congenital anomalies face significant stressors that impact their health-related quality of life (HRQL), which begin at diagnosis and continue through a prolonged NICU hospitalization and subsequent health needs.(2–4) We previously found that unlike parents of preterm infants, whose HRQL improves upon discharge home, parents of term infants do not follow the same pattern, and they continue to have low HRQL after NICU discharge.(5) Because many term infants admitted to the NICU are born with congenital anomalies, understanding the factors associated with lower parent HRQL before and after NICU discharge would help target appropriate family counseling and discharge planning.
HRQL is a multi-dimensional concept of the impact of a person’s health status on their overall quality of life.(6) For parents of infants with congenital anomalies, HRQL could be impacted by multiple factors. Infant illness severity has been associated with lower parent HRQL, but characteristics associated with congenital anomalies have not been evaluated previously.(4,5) Multiple factors may affect parent HRQL, including surgery in the neonatal period, multi-system involvement, long-term prognosis, complicating conditions such as growth restriction or prematurity, as well as demographic factors and post-discharge healthcare utilization.(5,7) The interaction of NICU illness, demographic factors, anomaly characteristics and post-discharge health utilization as it impacts parent HRQL for infants born with congenital anomalies has not yet been reported.
Our study objectives were to describe the association of neonatal anomaly characteristics with parent HRQL in the NICU and after discharge. We hypothesized that lower parent HRQL was associated with NICU illness, specific anomaly characteristics (such as surgically correctable vs. not), and that parents of infants with more post-NICU healthcare utilization needs would report lower HRQL after discharge.
Methods
This was a secondary analysis of two prospective cohort studies of parent-infant dyads hospitalized for at least 14 days in a single level IV NICU, the first from November 2016 to July 2017, and the second from September 2018 to March 2020.(5,8) Parent HRQL was measured in both, but the cohorts differed with respect to other measured outcomes.
Our level IV NICU admits infants of all gestational ages requiring intensive care born at a co-located birth hospital and receives patients transferred for medical and surgical evaluation. The NICU’s parent support resources include single patient rooms with video cameras, psychologists, case managers, social workers, and a family support coordinator. Our NICU admits all infants with congenital heart disease unless they require cardiac surgery or ECMO immediately after delivery. All infants with cardiac anomalies who require medical management alone are cared for in our NICU until discharge. Infants who require cardiac surgery are transferred to the cardiac ICU, typically 1–2 days prior to surgery and recover in the cardiac ICU, unless they are very preterm (<34 weeks), in which case they are transferred back to the NICU after recovery from cardiac surgery. For this study, we included all infants with major congenital anomalies, who had been identified by manual chart review for each of the original cohort studies. We included all infants admitted for NICU care either for management of the anomaly alone, or for another complicating factor in addition to the anomaly, for example, need for respiratory support or prematurity. Per the original study criteria, we excluded non–English speaking families, nonbiological parents who could not provide consent, infants previously discharged home, infants transferred to cardiac intensive care, or infants for whom death was imminent. Parents of multiples chose one child to enroll if both were eligible.
The primary outcomes were parent HRQL in the NICU and 3-months post-NICU discharge measured using the validated Pediatric Quality of Life (PedsQL) Family Impact Module.(9) This 36-item self-report tool assesses parent HRQL related to their child’s illness among 8 domains: physical, emotional, social, and cognitive functioning; communication; worry; family relationships; and daily activities. A 5-point Likert scale is used; mean scores are transformed to a 0–100 scale with higher scores indicating higher HRQL.
Because this cohort included infants with a diverse group of anomalies, we categorized them a-priori in different ways to assess how specific anomaly characteristics were associated with parent HRQL. Anomalies are traditionally classified by organ system and specific type in birth defect registries and medical textbooks (10–12). However, this classification does not necessarily correlate with what a baby might need in the NICU, or what the long-term outlook would be. Thus, we used a classification system that identified babies based on what they might need in the NICU (for example, surgery)(13), how that would impact NICU stay, and long-term prognosis as previously described.(13) Therefore, we categorized anomalies by need for neonatal surgery (neonatal surgical, non-neonatal surgical, non-surgical); by overall prognosis (moderate, severe, life-limiting) based on previously published research from our group; and by organ system (such as thoracic, cardiac etc.) with individual types of anomalies included separately within the organ system (such as CDH or gastroschisis). The definitions and examples of included anomalies are listed in Table I(available at www.jpeds.com).
Table 1:
Anomaly classification schemes and examples of included anomalies
| Anomaly classification schemes | |
|---|---|
| 1) Classification by prognosis*(≠) | Examples of anomalies included |
| Moderate = significant NICU course with either need for surgery in NICU or mechanical ventilation or LOS >14 days, but with overall good prognosis and good expected long term QOL based on previously reported data | Gastroschisis, CDH, CPAM, TEF, bowel atresias, bladder outlet obstruction, ventricular septal defect etc. Genetic conditions such as Pierre-Robin sequence, Turner’s syndrome etc. |
| Severe= expected long-term healthcare burdens that are typically life-long; possibility of needing repeat surgeries or procedures | MMC, cloacal exstrophy, hydrocephalus, single ventricle physiology, skeletal dysplasia etc. Genetic conditions such as Trisomy 21, CHARGE syndrome etc. |
| Life-limiting=anticipated neonatal or infant death; few long-term survivors | Trisomy 13 or 18, neonatal Marfan’s syndrome etc. |
| *For anomalies with a wide-ranging prognosis, we included them in the most anticipated prognostic category | |
| 2) Classification by need for surgery | |
| Neonatal surgical=will require surgery during the neonatal admission | Bowel atresias, congenital diaphragmatic hernia, tracheoesophageal fistula, gastroschisis, hypoplastic left ventricle etc. |
| Non-neonatal surgical= will require surgery at some point, but usually not during the neonatal admission | VSD, AV canal, cleft lip or cleft palate, club feet, CPAM, limb anomalies etc. |
| Non-surgical= do not/will not typically require surgery at any point or cannot be surgically corrected; infants in this group may have required surgery for reasons other than the anomaly, such as a G-tube for infants with severe developmental delays | Agenesis of corpus callosum, congenital myotonic dystrophy, and multiple other genetic conditions |
| 3) Classification by organ system/type of anomaly | |
| Genetic | Diagnosis associated with a genetic mutation or change e.g., trisomy 13, single gene disorders etc. |
| Associated with a syndrome | Subgroup of Genetic; constellation of anomalies associated with a known syndrome, such as CHARGE |
| Thoracic | TEF, CDH, CPAM, pulmonary lymphatic malformations |
| Cardiac | AV canal, large VSD, double outlet right ventricle, Ebstein’s anomaly, hypoplastic left ventricle etc. Minor anomalies such as ASD or PDA were not included. |
| Central Nervous System (CNS) | Myelomeningocele, aqueductal stenosis, absent septum pellucidum, agenesis of corpus callosum, ventriculomegaly, Chiari malformation, colpocephaly, Dandy Walker malformation/spectrum, encephalocele, holoprosencephaly, hydrocephalus, polymicrogyria, among others |
| Craniofacial | cleft lip/palate, eye, ear, or airway anomalies |
| Airway | Choanal atresia or stenosis, choanal stenosis, Pierre-Robin sequence, mandibular hypoplasia, microretrognathia |
| Eye | coloboma, cataract, retinitis pigmentosa, septo-optic dysplasia |
| Ear | Any ear, nose, throat malformations |
| Abdominal Wall Defect | Gastroschisis, omphalocele, cloacal exstrophy |
| Gastrointestinal | Bowel atresia (including duodenal, jejunal, ileal or colonic), Hirschsprung’s disease, imperforate anus |
| Renal/GU (Genitourinary) | Any kidney malformations or those affecting genitourinary system, such as cystic kidney disease, horseshoe kidney, polycystic kidney disease; or absent or micro penis, ambiguous genitalia, bladder outlet obstruction +/− bilateral hydronephrosis, hydronephrosis, posterior urethral valves |
| Musculoskeletal (MSK) | congenital myotonic dystrophy, limb anomalies, osteogenesis imperfecta, skeletal dysplasia, vertebral anomaly, club feet among others |
| Multiple anomaly complex | Multiple anomalies were present, either with or without an associated genetic or syndromic diagnosis; this term encompasses genetic anomalies associated with a syndrome as well as multiple anomalies that were not associated with any known syndrome or genetic diagnosis |
When 2 or more anomalies were present, patient was counted as having the more severe of the 2 anomalies (e.g., T21 with intestinal atresia would be counted as ‘severe’)
When 2 or more anomalies were present at least one of which was neonatal surgical, then the patient was counted as having a neonatal surgical anomaly
CDH= congenital diaphragmatic hernia; CPAM= congenital pulmonary airway malformation; TEF=tracheoesophageal fistula; VSD=ventricular septal defect; AV canal= atrioventricular canal defect; ASD=atrial septal defect; PDA= patent ductus arteriosus
This classification was adapted from the following reference: Baker A, Lagatta J, Leuthner S, Acharya K. Does prenatal counseling for pregnancies complicated by multiple fetal abnormalities concord with postnatal outcomes? Prenatal Diagnosis. 2020;40(5):538–48.
Study procedures for identification of eligible subjects, enrollment, and data collection were similar in both cohorts. (5) Eligible parents were approached for consent by a research assistant; after enrollment, parents completed questionnaires using a tablet. Responses were entered directly into a secure database. (16) Upon enrollment, parents answered demographic questions including self-reported history of a mental health disorder and the PedsQL Family Impact Module. At discharge, we reviewed the chart for variables that would reflect NICU illness, comorbidities, and discharge medical needs across all anomalies. Three months after discharge, healthcare utilization was evaluated using chart review. Parents were contacted 3 months after discharge to repeat the PedsQL Family Impact Module and confirm healthcare utilization; this post-NICU assessment occurred using secure electronic questionnaires (16), by phone or in person depending on parent preference.
Statistical Analyses
We calculated that a sample size of 111 patients would be sufficient to test a change in HRQL of 5 points between pre- and post-NICU discharge with 80% power at a probability level of 0.05. Anomaly characteristics were comparable between the baseline and the 3-month sample. We compared demographics, infant illness, and post-NICU healthcare use among infants with anomalies. Next, we compared NICU and 3-month parent HRQL scores by demographics, anomaly type, infant illness, and post-NICU healthcare use. Between-group comparisons were performed with Kruskal Wallis or Wilcoxon rank-sum tests, chi-squared tests, or Fisher’s exact tests as appropriate; within-group changes in HRQL were compared with paired-samples Wilcoxon signed-rank tests. Multivariable regression was used to assess the impact of multiple predictors on baseline HRQL and 3-month HRQL separately. We included variables with a p-value of <0.2 in bivariate analysis in the model. For 3-month HRQL, the baseline HRQL was included as a predictor. This study was approved by the Institutional Review Board at the Children’s Hospital of Wisconsin.
Results
From July 2016-Feb 2020, a total of 514 parent-infant dyads were enrolled in 2 separate cohort studies. The first study enrolled 214 parents and the second study enrolled 300 parents. From those cohorts, 166 infants (32%) had a major congenital anomaly and were included in this study; 154 of those parents were eligible for follow-up (8 infants died in NICU or prior to 3-month follow-up post-discharge; 4 were still in NICU at the completion of study period). Of the 154 eligible parents, 124 (81%) completed the 3-month interview. Eighty-eight percent of enrolled parents were mothers.
The most common anomalies were genetic (n=53, 32%), followed by thoracic, cardiac, craniofacial, and abdominal wall defects (Table II). Based on our prognostic classification, 106 (64%) of the anomalies were classified as moderate severity, 53 (32%) were severe, and 7 (4%) were life-limiting. The majority of anomalies (102, 61%) were classified as neonatal surgical.
Table 2.
Bivariate analysis of parent HRQL and parent, infant, illness, and anomaly characteristics
| NICU (n=166) | 3-month (n=124) | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | n | % | Median (IQR) | p | n | % | Median (IQR) | p |
| Parent characteristics | ||||||||
| Age, median (IQR) | ||||||||
| <=18 | 3 | 2% | 82(80–87) | 0.16 | 3 | 2% | 88(68–89) | 0.07 |
| 19–25 | 37 | 22% | 64(50–81) | 28 | 23% | 70(61–82) | ||
| 26–35 | 95 | 57% | 67(55–76) | 70 | 56% | 63(52–80) | ||
| >=36 | 31 | 19 % | 68(60–77) | 23 | 19 % | 70(62–89) | ||
| Race/Ethnicity | ||||||||
| Black | 30 | 18% | 66(58–74) | 0.08 | 19 | 15% | 68(57–75) | 0.14 |
| White | 115 | 69% | 66(52–77) | 92 | 74% | 67(52–82) | ||
| Hispanic | 8 | 5% | 71(48–77) | 5 | 4% | 68(54–80) | ||
| Asian | 4 | 2% | 80(77–84) | 3 | 2% | 84(80–89) | ||
| Other | 9 | 5% | 76(63–90) | 5 | 4% | 83 (71–94) | ||
| Has a car | ||||||||
| Yes | 137 | 83% | 66(54–76) | 0.07 | 104 | 84% | 68(56–82) | 0.71 |
| No | 29 | 17 % | 74(61–82) | 20 | 16 % | 71(53–84) | ||
| History of mental health disorder* | ||||||||
| Yes | 46 | 28% | 56(46–68) | <0.001 | 33 | 27% | 64(50–77) | 0.20 |
| No | 80 | 48% | 69(59–78) | 59 | 48% | 75(57–88) | ||
| Parent psychology consult in NICU | ||||||||
| Yes | 22 | 13% | 50(42–62) | 0.06 | 14 | 11% | 56(48–59) | <0.01 |
| No | 144 | 87% | 68(65–70) | 110 | 89% | 69(57–84) | ||
| Number of siblings (for child) | ||||||||
| 0 | 53 | 32% | 69(55–81) | 0.28 | 41 | 33% | 71(58–84) | 0.15 |
| 1 | 55 | 33% | 64(52–74) | 41 | 33% | 67(49–75) | ||
| 2 or more | 58 | 35% | 68(57–77) | 42 | 34% | 69(56–83) | ||
| NICU illness characteristics | NICU | 3-month | ||||||
| Gestational age, median (IQR) | n | % | Median (IQR) | p | n | % | Median (IQR) | p |
| <=28 weeks | 7 | 4% | 51(49–76) | 0.09 | 6 | 5% | 68(44–72) | 0.71 |
| 29–36 weeks | 82 | 49% | 65(53–76) | 58 | 47% | 71(56–84) | ||
| >=37 weeks | 77 | 46% | 68(60–78) | 60 | 48% | 68(56–82) | ||
| Gender | ||||||||
| Male | 91 | 55% | 67(52–80) | 0.98 | 67 | 54% | 64(52–83) | 0.17 |
| Female | 75 | 45% | 67(58–76) | 57 | 46% | 71(62–82) | ||
| Number of consultants * | ||||||||
| 0–1 | 25 | 15% | 77(64–89) | 0.01 | 17 | 14% | 80(63–87) | 0.05 |
| >=2 | 141 | 85% | 66(53–76) | 107 | 86% | 67(54–81) | ||
| Palliative care consulted | ||||||||
| Yes | 16 | 10% | 57(51–65) | 10 | 8% | 62(46–70) | ||
| No | 150 | 90% | 68(56–78) | 0.07 | 114 | 92% | 68(57–83) | 0.08 |
| Received mechanical ventilation | ||||||||
| Yes | 101 | 61% | 66(53–77) | 0.14 | 75 | 60% | 67(54–81) | 0.06 |
| No | 65 | 39 % | 68(60–80) | 49 | 40% | 75(59–87) | ||
| Received vasopressors, % | ||||||||
| Yes | 36 | 22% | 68(55–80) | 0.48 | 27 | 22% | 74(62–84) | 0.18 |
| No | 130 | 78% | 66(55–76) | 97 | 78% | 67(56–82) | ||
| Total length of hospital stay | ||||||||
| <=28 days | 46 | 28% | 69(62–80) | 0.04 | 33 | 27% | 71(61–85) | 0.15 |
| 29 days–60 days | 70 | 42% | 68(55–81) | 56 | 45% | 67(53–83) | ||
| >61 days | 50 | 30 % | 64(51–72) | 35 | 28% | 68(48–77) | ||
| Anomaly-specific characteristics | ||||||||
| Classification by need for surgery | ||||||||
| Neonatal surgical | 102 | 61% | 69 (57–80) | 0.13 | 82 | 66% | 71(57–84) | 0.05 |
| Non-neonatal surgical | 26 | 16% | 63(52–69) | 19 | 15% | 58(48–69) | ||
| Non-surgical | 38 | 23 % | 65 (53–75) | 23 | 19 % | 68(58–80) | ||
| Classification by prognosis | ||||||||
| Moderate | 106 | 64% | 68 (59–77) | 0.46 | 81 | 65% | 68(56–84) | 0.88 |
| Severe | 53 | 32% | 66(52–80) | 41 | 33% | 68(53–82) | ||
| Life-limiting | 7 | 4% | 60 (44–75) | 2 | 2% | 63(58–68) | ||
| Classification by organ system | ||||||||
| Genetic | 53 | 32% | 64(52–75) | 0.14 | 35 | 28% | 66(49–81) | 0.16 |
| Associated with a syndrome | 25 | 15% | 62(53–77) | 0.85 | 18 | 15% | 62(39–77) | 0.76 |
| Thoracic | 30 | 18% | 72(59–85) | 0.04 | 24 | 19% | 69(56–90) | 0.52 |
| TEF/EA | 13 | 8% | 67(55–86) | 0.69 | 11 | 9% | 73(58–82) | 0.71 |
| CDH | 12 | 7% | 76(68–80) | 0.05 | 11 | 9% | 64(53–91) | 0.76 |
| CPAM | 3 | 2% | 70 (52–88) | 0.75 | 1 | 1% | 92 | 0.16 |
| Pulmonary lymphatic malformation | 2 | 1% | 75 (65–88) | 0.25 | 1 | 1% | 58 | 0.50 |
| Cardiac | 29 | 17% | 60(46–70) | 0.02 | 20 | 16% | 59(48–70) | 0.02 |
| CNS | 27 | 16% | 62(51–75) | 0.29 | 21 | 17% | 67(51–74) | 0.36 |
| Craniofacial | 27 | 16% | 68(58–76) | 0.61 | 19 | 15% | 67(48–82) | 0.55 |
| Airway | 15 | 9% | 67(58–74) | 0.95 | 11 | 9% | 67(49–89) | 0.83 |
| Cleft lip/palate | 12 | 7% | 61(54–74) | 0.64 | 9 | 7% | 56(48–81) | 0.23 |
| Eye | 6 | 4% | 74(65–86) | 0.12 | 4 | 3% | 74(69–90) | 0.24 |
| Ear | 3 | 2% | 73(28–77) | 0.85 | 2 | 2% | 56 (24–89) | 0.79 |
| Abdominal wall defect | 25 | 15% | 69(60–81) | 0.43 | 21 | 17% | 71(62–84) | 0.15 |
| Gastroschisis | 20 | 12% | 68(50–81) | 0.88 | 16 | 13% | 72(62–84) | 0.24 |
| Omphalocele | 4 | 2% | 81 (70–89) | 0.06 | 4 | 3% | 75(68–94) | 0.22 |
| Cloacal exstrophy | 1 | 1% | 73 | 0.60 | 1 | 1% | 56 | 0.38 |
| Gastrointestinal | 18 | 11% | 70(58–81) | 0.39 | 13 | 10% | 69(57–87) | 0.52 |
| Intestinal atresias | 12 | 7% | 65(60–76) | 0.75 | 7 | 6% | 73(57–85) | 0.53 |
| Hirschsprung’s disease | 4 | 2% | 83(60–94) | 0.12 | 4 | 3% | 88(71–93) | 0.05 |
| Imperforate anus | 4 | 2% | 76(52–83) | 0.47 | 4 | 3% | 62(33–77) | 0.37 |
| Renal/GU | 15 | 9% | 69(58–75) | 0.98 | 8 | 6% | 66(33–86) | 0.40 |
| Musculoskeletal (MSK) | 13 | 8% | 59(52–66) | 0.10 | 9 | 7% | 62(47–73) | 0.24 |
| Multiple anomaly complex | 40 | 24% | 64(56–77) | 0.62 | 9 | 7% | 64(49–80) | 0.24 |
| Healthcare utilization at NICU discharge | ||||||||
| Home oxygen | ||||||||
| Yes | 31 | 25% | 67(49–77) | 0.20 | ||||
| No | 93 | 75% | 69(57–84) | |||||
| G Tube | ||||||||
| Yes | 48 | 39% | 63(48–81) | |||||
| No | 76 | 61 % | 69(58–84) | 0.06 | ||||
| Emergency Department visits | ||||||||
| None | 69 | 56% | 69(57–85) | 0.05 | ||||
| >=1 | 55 | 44% | 67(52–77) | |||||
| Hospital readmissions | ||||||||
| None | 68 | 55% | 71(58–84) | 0.06 | ||||
| >=1 | 56 | 45% | 66(49–80) | |||||
contains missing values; G tube= gastrostomy tube
CDH= congenital diaphragmatic hernia; CPAM/CCAM= congenital pulmonary airway malformation; TEF/EA=tracheoesophageal fistula; MSK=musculoskeletal
For parent and infant characteristics, only variables with a p-value of <0.2 in either the initial, 3-month, or pre-post difference are shown. p-values <=0.05 are in bold font. Other variables that were tested but not found to be significant were parent education, distance from hospital, single parent household, insurance type, presence of a prenatal diagnosis, adults nearby to care for children, birth weight, multiple gestation, number of surgeries, and mode of delivery.
Table II shows bivariable associations between parent demographics, NICU illness, and anomaly-specific characteristics with parent HRQL at baseline and at 3-month follow-up. Parent history of a mental health disorder, consultation by multiple specialists, and longer hospital stay were associated with lower HRQL in the NICU. Presence of a neonatal surgical anomaly was associated with higher parent HRQL at 3 months. Anomaly prognostic classification did not impact parent HRQL. Post-NICU healthcare utilization was also associated with 3-month HRQL differences; parents of infants with G-tubes, and those who had hospital readmissions had lower HRQL.
Table III shows HRQL subdomain scores in the NICU and 3 months. In the NICU, parents of infants with anomalies scored the lowest on the subdomains of worry and daily activities. From the NICU to 3 months, daily activity scores significantly declined (−8 points), whereas emotional functioning scores significantly improved (+10 points).
Table 3.
HRQL subdomain scores for parents of infants with congenital anomalies at baseline and at 3-month follow-up
| Subdomain | NICU (n=166) | 3 Months (n=124) | Change | p-value for change |
|---|---|---|---|---|
| Physical functioning | 62 (50–75) | 67 (50–75) | 0 (−8 to +12) | 0.58 |
| Emotional functioning | 65 (45–75) | 72 (55–90) | +10 (−5 to+20) | <0.001 |
| Social functioning | 75 (56–87) | 69 (50–94) | 0 (−14 to +15) | 0.12 |
| Cognitive functioning | 70 (55–90) | 72 (55–90) | 0 (−14 to +15) | 0.92 |
| Communication | 67 (50–83) | 67 (50–83) | 0 (−8 to +8) | 0.92 |
| Worry | 60 (45–75) | 65 (50–80) | 0 (−10 to +15) | 0.16 |
| Daily activities | 58 (42–75) | 50 (33–75) | −8 (−17 to +8) | <0.01 |
| Family relationships | 80 (65–95) | 80 (60–100) | 0 (−10 to +5) | 0.18 |
Table 3 shows median (interquartile range) scores for parents in the NICU and at 3 months after discharge; “change” is the median same-parent change score comparing 3 months to NICU enrollment. P values are measured by paired Wilcoxon rank-sum tests. P-values <0.05 are in bold font
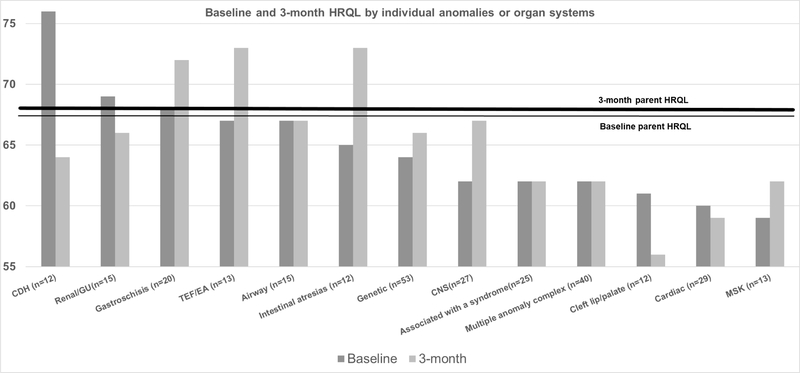
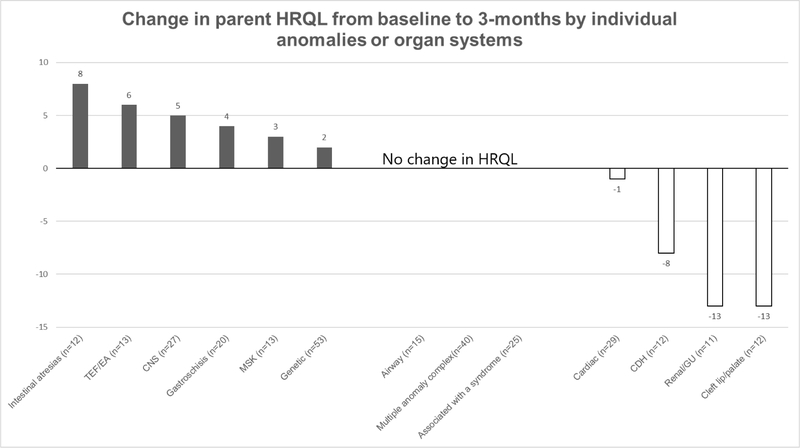
Figure 1 shows parent HRQL associated with specific types of anomalies or organ systems with at least 10 patients for assessment. During the NICU stay, parents of infants with CDH had higher scores than parents of infants with other anomalies; parents of infants with musculoskeletal and cardiac anomalies had lower than median NICU scores. At 3 months post-discharge, infants with CDH had lower scores, and infants with TEF, intestinal atresias, and gastroschisis had higher scores. When comparing parents in the NICU versus at 3 months (Figure 2), parents of infants with intestinal atresias, TEF/EA, neurologic anomalies showed improvement over time, whereas parents of infants with CDH, genitourinary anomalies and cleft lip/palate showed decreased HRQL after discharge. Parents of infants with multiple anomaly complex showed no change.
Figure 1. Baseline and 3-month HRQL by individual anomalies or organ systems.
The figure presents the median HRQL score on the Y-axis
Only anomalies with >10 patients are shown in figure
CDH=congenital diaphragmatic hernia
TEF/EA= tracheo-esophageal fistula/esophageal atresia
CNS=central nervous system
*p<0.05 for difference between baseline and 3-month post NICU discharge HRQL scores
Figure 2. Change in parent HRQL from baseline to 3-months by individual anomalies or organ systems.
Only anomalies with >10 patients are shown in figure
*p<0.05 for change in HRQL score from baseline to 3-month post NICU discharge
Table IV (available at www.jpeds.com) shows results of a multivariable regression model assessing the effect of multiple predictors on baseline and 3-month parent HRQL. Factors associated with a lower parent HRQL in the NICU were parent history of mental health disorder (9 points lower) and multiple consultants involved in a patient’s care (10 points lower). At 3 months, hospital readmissions were associated with an 8-point decrease in parent HRQL compared with their own HRQL in the NICU, adjusted for other covariates.
Table 4.
Multivariable regression model showing predictors of baseline and 3-month parent HRQL
| Baseline HRQL | ||||
|---|---|---|---|---|
| Variable | Coefficient | 95% LCI | 95% UCI | p-value |
| Parent age | −1.25 | −0.65 | 0.4 | 0.64 |
| Race | ||||
| Black | referent | |||
| White | −3.33 | −11.16 | 4.5 | 0.401 |
| Hispanic | 3.2 | −12.4 | 18.8 | 0.686 |
| Asian | 10.6 | −7.06 | 28.3 | 0.236 |
| Other | 6.66 | −5.76 | 19.09 | 0.29 |
| Has a car | 1.24 | −7.59 | 10.08 | 0.781 |
| History of mental health disorder | −9.2 | −15.1 | −3.2 | 0.003 |
| Anomaly classification by surgery | ||||
| Neonatal surgical | referent | |||
| Non-neonatal surgical | −2.15 | −10.1 | 5.8 | 0.593 |
| Non-surgical | −0.9 | −7.5 | 5.6 | 0.779 |
| Gestational age | ||||
| <=28 weeks | referent | |||
| 29–36 weeks | 2.7 | −12.3 | 17.7 | 0.723 |
| >=37 weeks | 7.4 | −8.6 | 23.5 | 0.362 |
| Multiple consultants | −10.3 | −18.4 | −2.2 | 0.013 |
| Palliative care involved | −8.3 | −18.7 | −2.2 | 0.1 15 |
| Psychology consult in the NICU | ||||
| Mechanical ventilation in the NICU | −2.9 | −9.2 | 3.5 | 0.377 |
| LOS | 0.01 | −0.08 | 0.11 | 0.825 |
| 3-month HRQL | ||||
| Enrollment HRQL | 0.83 | 0.58 | 1.09 | <0.001 |
| (every 1 point) | ||||
| Gestational age | ||||
| <=28 weeks | referent | |||
| 29–36 weeks | 10.8 | −7.9 | 9.8 | 0.25 |
| >=37 weeks | 8.1 | −10.7 | 26.9 | 0.39 |
| Anomaly classification by surgery(1) | ||||
| Neonatal surgical | referent | |||
| Non-neonatal surgical | −2 | −13.8 | 9.7 | 0.73 |
| Non-surgical | −0.1 | −10.9 | 10.5 | 0.97 |
| Discharged with G-tube | 1.2 | −7 | 9.6 | 0.76 |
| Hospital readmissions | −7.9 | −15.6 | −0.2 | 0.04 |
| Discharged with home oxygen | −2.1 | −11.8 | 7.6 | 0.67 |
LOS=length of stay; G-tube= gastrostomy tube
Discussion
In our prospective single-center cohort study of infants with anomalies requiring NICU care we found that parents of infants admitted with major anomalies to the NICU report low HRQL, both at baseline, and after NICU discharge. Parents of infants with neonatal surgical anomalies had higher HRQL, and anomaly prognosis did not affect parent HRQL in the NICU or at follow-up. The main factors associated with lower parent HRQL were poor parent mental health, need for multiple consultants, presence of a syndrome, and need for continued medical care.
Parents of infants admitted with major anomalies to a level IV NICU reported low overall HRQL, both in the NICU (median 67) and 3 months after discharge (median 68). As reference, the average HRQL reported in a healthy pediatric population is about 84 and in those with chronic disease is 74. (17–19) In our earlier study of infants without anomalies admitted to the NICU, the baseline HRQL was 70 and the 3-month HRQL was 75 (5). In our current cohort of infants with anomalies, at 3 months post-NICU discharge, the overall HRQL of parents in this cohort did not significantly improve. When we examined subdomain scores, we found that parents of infants with anomalies report more worry and significantly more difficulty with performing daily activities (for example, daily activities take more time and effort, difficulty finding time and energy to finish household tasks), and that this subdomain is negatively affected upon discharge home. The higher proportion of parents reporting a mental health history in our parent cohort may also affect these subdomain scores. In contrast to our findings in parents of extremely preterm infants who report an improvement in most subdomain scores upon discharge home, parents of infants with anomalies do not show such an improvement (5). Research on parents with older children with certain anomalies has also shown effects on their physical and emotional functioning, although this finding is not consistent across studies.(20) Our findings suggest that parents of infants admitted with anomalies are a more vulnerable population compared with parents of infants requiring NICU admission for prematurity.
We explored different anomaly classification methods (such as by prognosis, organ system etc.) as predictors of parent HRQL. Parents of infants with neonatal surgical anomalies reported higher NICU HRQL and those with non-surgical anomalies reported lower HRQL. Parents of infants with surgical anomalies such as intestinal atresias, TEF, or gastroschisis had significantly better quality of life, but this was not true for parents of infants with CDH, omphalocele, or imperforate anus. We speculate that post-discharge health care needs in these patients could at least partly explain the lower parent quality of life after discharge. Amin et al showed that in infants with CDH, ongoing need for a feeding tube is associated with lower quality of life.(17), which was also seen in our study population. Ongoing care requirements (such as in omphalocele or care of a colostomy in infants with an imperforate anus) may pose additional burdens on families.(17,18) In addition, for some anomalies such as CDH, the lower HRQL may reflect parents recognizing that their baby has a serious chronic medical condition. Some of these families could be better supported by incorporating parent education and better preparation for the care needs after the transition to home. Parents of infants with non-neonatal surgical anomalies such as cleft lip or palate also reported lower quality of life upon discharge home. This may be due to ongoing feeding difficulties at home, need for feeding assistance, association with a syndrome of multiple anomalies, or that the defect has not yet been repaired and is physically more obvious. The reasons for lower parent quality of life for some of these anomalies need to be further explored. We acknowledge that HRQL is a dynamic construct that changes over time; parent attitudes about their child’s quality of life likely change with time, and the 3-month time frame of our study does not reflect more long-term changes. For many anomalies, HRQL has been shown to be worst for the youngest children (<4 years of age) and improves over time. (23,24) However, our short-term time frame does provide perspective on the well-being of these families as they transition home and should inform communication with the primary care providers that take over their care following NICU discharge.
The type of anomaly and complexity of care (number of consultants, length of stay, palliative care involvement) were influential in determining parent HRQL, but the two are likely interrelated factors. For example, infants with genetic anomalies had longer length of stay, more consultants, and were more likely to carry a worse prognosis. Whether the anomaly itself or its associated medical complexity contributes to lower parent HRQL remains to be explored. We speculate that parents of infants with certain surgical anomalies may derive hope in their child’s problems as temporary and able to be ‘fixed’, whereas those with non-surgical anomalies may perceive that problems are more permanent or not ‘fixable’. Moreover, infants with surgical anomalies tended to have fewer consultants involved, as opposed to a patient with a more complex genetic diagnosis where multiple specialists may be involved. Prior work on parent experience in the NICU for infants born with anomalies has shown that parents often feel overwhelmed, and desire more empathetic listening and better communication.(25) Parents with a history of mental health concerns and parents of premature infants with anomalies may be especially vulnerable to poor quality of life. Addressing the needs of the baby and the family as a whole, rather than relegating individual organ systems to multiple specialists may improve parent quality of life.
Overall, this cohort of patients had significant home health care needs and post-NICU healthcare utilization. 25% of infants were discharged with home oxygen and 40% were discharged home with a G-tube. More than 40% of infants were readmitted to the hospital within the study period. Parents of children with G-tubes or those with hospital readmissions had lower 3-month HRQL.
Parent quality of life is multifactorial, and likely driven by factors beyond what we report in our study. Some families may easily adapt to the need for home medical equipment, and others may struggle. For some children, these needs may be temporary, and for others, they may be lifelong. Regardless, fostering early independence with care, better education for families being discharged with home medical equipment, and setting realistic expectations in terms of need for future healthcare utilization might better prepare families to cope with their child’s health care needs. In addition, a better understanding of what outcomes are important to families of infants with anomalies is vital for effective communication.
We acknowledge certain limitations to our study. As a single-center study, certain anomalies were underrepresented. Parents of infants who were discharged home prior to 14 days of NICU stay were excluded. Although the majority of anomalies represented in our cohort would necessitate a longer NICU stay, it is possible that infants with more favorable prognoses were excluded. We did not have a large enough sample to fully evaluate the effect of disease severity on HRQL within each anomaly category. Although we represent quantitative differences in quality of life, we did not explore the reasons why some parents reported worse quality of life. We measured post-NICU HRQL at 3-months but do not know long-term quality of life for these families. Despite these limitations, this is one of the first studies to explore parent quality of life for infants with anomalies during the NICU and post-NICU period and factors associated with quality of life.
In conclusion, parents of infants with anomalies admitted to NICUs represent a population at risk for lower quality of life in the NICU and after discharge home. Complexity of NICU care and post-NICU healthcare needs likely place burdens on families that affect their quality of life. These parents could be targeted earlier in their NICU course by the NICU team with education, hands-on training, and anticipatory guidance as to what to expect after discharge home. Future research should focus on qualitative evaluation of parental quality of life in this population as well as large, multi-institute analysis of data for specific congenital anomalies.
Funding/Support:
Supported by the National Institutes of Health (K23HL136525 [to J.L.]) and a Medical College of Wisconsin Presidential Faculty Scholar Award (to J.L.)
Abbreviations:
- NICU
neonatal intensive care unit
- HRQL
health-related quality of life
Footnotes
Data sharing statement: Data from the study are not available for sharing.
Conflicts of Interest: The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiner J. How Infants Die in the Neonatal Intensive Care Unit: Trends From 1999 Through 2008. Arch Pediatr Adolesc Med. 2011. Jul 1;165(7):630. [DOI] [PubMed] [Google Scholar]
- 2.Ratliffe CE, Harrigan RC, Haley J, Tse A, Olson T. Stress in Families with Medically Fragile Children. Issues in Comprehensive Pediatric Nursing. 2002. Jan 1;25(3):167–88. [DOI] [PubMed] [Google Scholar]
- 3.Kuhlthau K, Hill KS, Yucel R, Perrin JM. Financial Burden for Families of Children with Special Health Care Needs. Matern Child Health J 2005. Jun;9(2):207–18. [DOI] [PubMed] [Google Scholar]
- 4.Golfenshtein N, Hanlon AL, Deatrick JA, Medoff-Cooper B. Parenting Stress in Parents of Infants With Congenital Heart Disease and Parents of Healthy Infants: The First Year of Life. Comprehensive Child and Adolescent Nursing. 2017. Oct 2;40(4):294–314. [DOI] [PubMed] [Google Scholar]
- 5.McAndrew S, Acharya K, Westerdahl J, Brousseau DC, Panepinto JA, Simpson P, et al. A Prospective Study of Parent Health-Related Quality of Life before and after Discharge from the Neonatal Intensive Care Unit. The Journal of Pediatrics. 2019. Oct 1;213:38–45.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guyatt GH, Feeny DH, Patrick DL. Measuring Health-Related Quality of Life. Ann Intern Med. 1993. Apr 15;118(8):622–9. [DOI] [PubMed] [Google Scholar]
- 7.Graham RJ, Rodday AM, Weidner RA, Parsons SK. The Impact on Family of Pediatric Chronic Respiratory Failure in the Home. The Journal of Pediatrics. 2016. Aug 1;175:40–6. [DOI] [PubMed] [Google Scholar]
- 8.Lagatta JM, Uhing M, Acharya K, Lavoie J, Rholl E, Malin K, et al. Actual and Potential Impact of a Home Nasogastric Tube Feeding Program for Infants Whose Neonatal Intensive Care Unit Discharge Is Affected by Delayed Oral Feedings. The Journal of Pediatrics. 2021. Jul 1;234:38–45.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varni JW, Sherman SA, Burwinkle TM, Dickinson PE, Dixon P. The PedsQL™ Family Impact Module: Preliminary reliability and validity. Health and Quality of Life Outcomes. 2004. Sep 27;2(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC. 5.1 International Classification of Diseases [Internet]. Centers for Disease Control and Prevention. 2018. [cited 2021 Oct 27]. Available from: https://www.cdc.gov/ncbddd/birthdefects/surveillancemanual/facilitators-guide/module-5/mod5-1.html [Google Scholar]
- 11.Fetology : Diagnosis and management of the fetal patient second edition - Diana W. Bianchi by Diana W. Bianchi: Used: Like New (2010) | Book Hèmisphères [Internet]. [cited 2021 Oct 27]. Available from: https://www.abebooks.com/9780071442015/Fetology-Diagnosis-management-fetalpatient-0071442014/plp
- 12.CDC. What are Birth Defects? | CDC [Internet]. Centers for Disease Control and Prevention. 2020. [cited 2021 Oct 27]. Available from: https://www.cdc.gov/ncbddd/birthdefects/facts.html [Google Scholar]
- 13.Acharya K, Leuthner S, Clark R, Nghiem-Rao TH, Spitzer A, Lagatta J. Major anomalies and birthweight influence NICU interventions and mortality in infants with trisomy 13 or 18. J Perinatol. 2017. Apr;37(4):420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker A, Lagatta J, Leuthner S, Acharya K. Does prenatal counseling for pregnancies complicated by multiple fetal abnormalities concord with postnatal outcomes? Prenatal Diagnosis. 2020;40(5):538–48. [DOI] [PubMed] [Google Scholar]
- 15.Leuthner SR. Palliative care of the infant with lethal anomalies. Pediatr Clin North Am. 2004. Jun;51(3):747–59, xi. [DOI] [PubMed] [Google Scholar]
- 16.About – REDCap [Internet]. [cited 2021 Jun 9]. Available from: https://projectredcap.org/about/
- 17.Amin R, Knezevich M, Lingongo M, Szabo A, Yin Z, Oldham KT, et al. Long-term Quality of Life in Neonatal Surgical Disease. Annals of Surgery. 2018. Sep;268(3):497–505. [DOI] [PubMed] [Google Scholar]
- 18.Varni JW, Burwinkle TM, Seid M, Skarr D. The PedsQL™* 4.0 as a Pediatric Population Health Measure: Feasibility, Reliability, and Validity. Ambulatory Pediatrics. 2003. Nov 1;3(6):329–41. [DOI] [PubMed] [Google Scholar]
- 19.Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JEC, Heffer RW, et al. The PedsQL™ Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011. Feb;20(1):45–55. [DOI] [PubMed] [Google Scholar]
- 20.Glinianaia SV, Embleton ND, Rankin J. A systematic review of studies of quality of life in children and adults with selected congenital anomalies. Birth Defects Research Part A: Clinical and Molecular Teratology. 2012;94(7):511–20. [DOI] [PubMed] [Google Scholar]
- 21.McCann D, Bull R, Winzenberg T. The daily patterns of time use for parents of children with complex needs: A systematic review. J Child Health Care. 2012. Mar 1;16(1):26–52. [DOI] [PubMed] [Google Scholar]
- 22.Javalkar K, Rak E, Phillips A, Haberman C, Ferris M, Van Tilburg M. Predictors of Caregiver Burden among Mothers of Children with Chronic Conditions. Children. 2017. May;4(5):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deurloo JA, Ekkelkamp S, Hartman EE, Sprangers MAG, Aronson DC. Quality of Life in Adult Survivors of Correction of Esophageal Atresia. Archives of Surgery. 2005. Oct 1;140(10):976–80. [DOI] [PubMed] [Google Scholar]
- 24.Poley MJ, Stolk EA, Tibboel D, Molenaar JC, Busschbach JJV. Short term and long term health related quality of life after congenital anorectal malformations and congenital diaphragmatic hernia. Archives of Disease in Childhood. 2004. Sep 1;89(9):836–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Catlin A, Áskelsdóttir B, Conroy S, Rempel G. From Diagnosis to Birth: Parents’ Experience When Expecting a Child With Congenital Anomaly. Advances in Neonatal Care. 2008. Dec;8(6):348–54. [DOI] [PubMed] [Google Scholar]