Figure 6. Loss of BMAL1 function in NAc astrocytes disrupts NAc metabolic homeostasis.
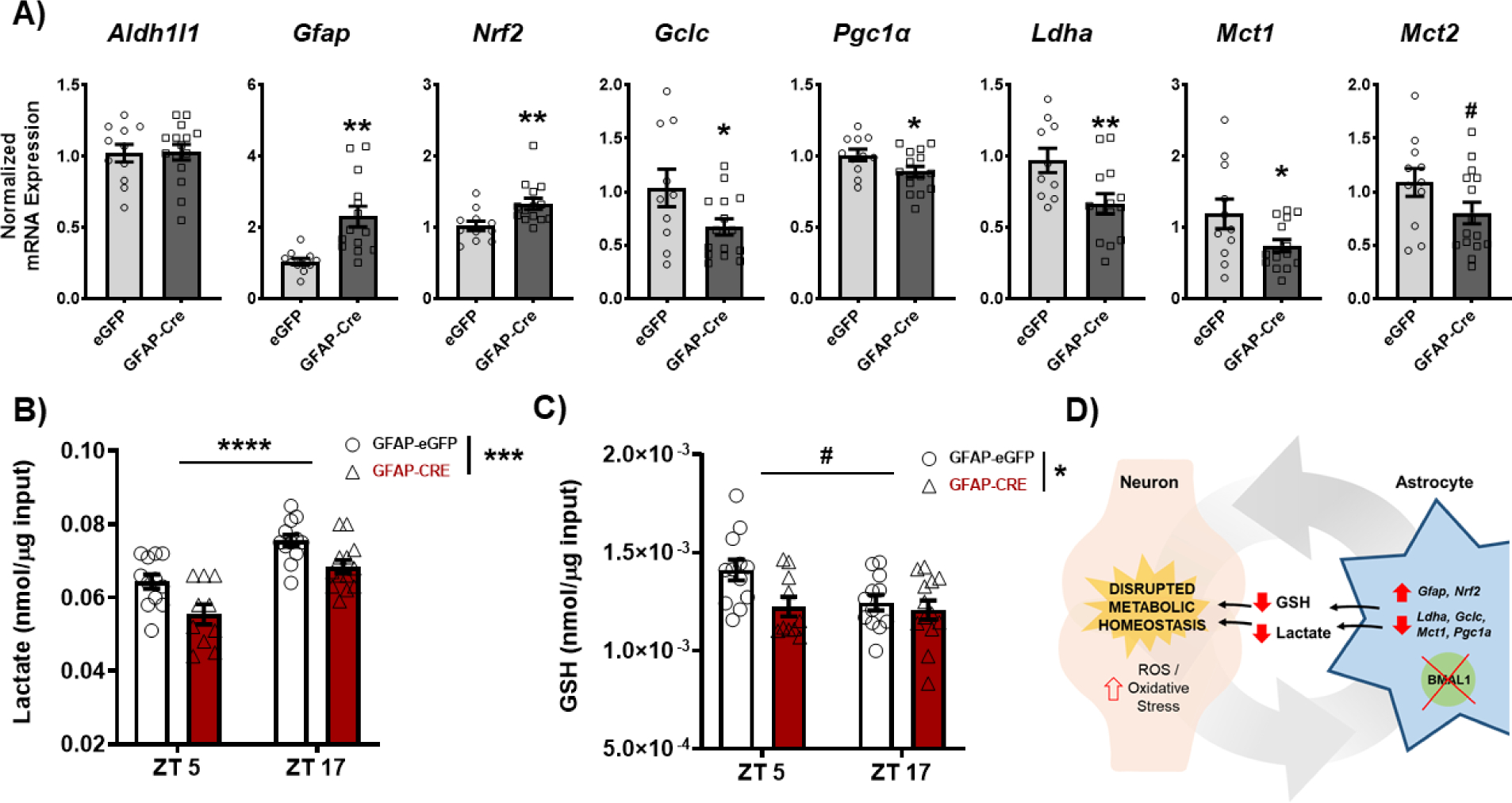
NAc tissue from both BMFL mice expressing NAc-specific GFAP-Cre or eGFP control was processed through a range of molecular assays assessing factors relevant for regulation of cellular metabolic state. (A) Through reverse transcriptase quantitative PCR (RT-qPCR), a panel of metabolic genes was assessed in the whole NAc of GFAP-Cre and eGFP control mice (ZT4–6). While there were no differences in the astrocyte marker Aldh1l1 (t(24) = 0.09, p=0.92), GFAP-Cre mice showed significantly elevated expression of Gfap (t(23) = 3.74, ** p=0.001) and Nrf2 (t(23) = 2.87, ** p=0.008), markers of astrocyte activation and oxidative stress antioxidant response. GFAP-Cre mice also showed significant reductions in genes relevant for glutathione (GSH) production (Gclc; t(23) = 2.15, * p<0.05), mitochondrial biogenesis (Pgc1a; t(24) = 2.13, * p<0.05), and lactate synthesis and shuttling (Ldha, t(22) = 2.75, * p=0.01; Mct1, t(24) = 2.18, * p<0.05; & Mct2, t(24) = 1.77, # p=0.08). (B) Moreover, loss of BMAL1 function in NAc astrocytes significantly reduces the concentration of lactate (Time: F(1, 43) = 35.76, ****p<0.0001; Virus: F(1, 43) = 15.79, *** p=0.0003; Interaction: F (1, 43) = 0.20, p=0.65) and (C) GSH (Time: F(1, 43) = 3.56, # p=0.06; Virus: F1, 43) = 5.31, * p=0.02; Interaction: F(1, 43) = 2.36, p=0.13) in the whole NAc across time of day. (D) The effects on key metabolic genes, lactate, and GSH are hypothesized to disrupt neurometabolic homeostasis, potentially leading to the downstream effects on NAc-regulated behavior. Gene expression normalized to 18s. Mean± SEM. N=10–15. # p=0.06; * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
