Figure 3.
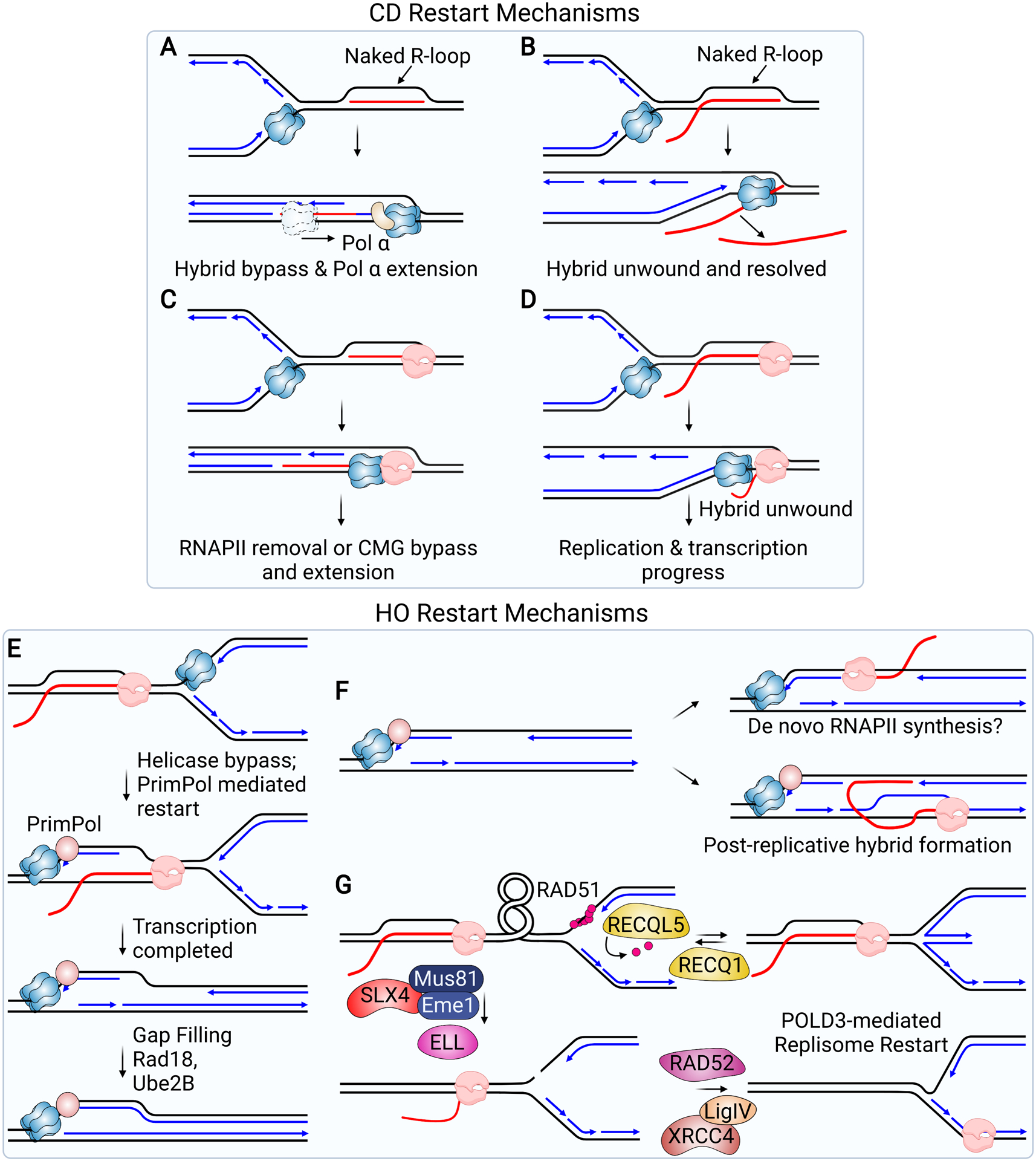
Models for the Restart of Replication Forks Stalled at R-loops. (A-D) illustrate potential bypass mechanisms in the CD orientation. (A) The CMG helicase translocates over and bypasses a “naked” hybrid when the 5’-end of the RNA strand is annealed to the DNA. Once the hybrid is bypassed, the RNA strand can be extended by Pol α. (B) The CMG helicase unwinds a hybrid when the 5’-end of the RNA strand is exposed as a flap, allowing fork progression to continue. (C) CMG translocation over the hybrid would lead to its arrest at RNAP. Fork progression at this point would require CMG to bypass the stalled RNAP or for RNAP to be removed. (D) CMG unwinding of a hybrid bound to RNAP would allow RNAP to continue and the replication fork to progress behind transcription. (E-G) illustrate potential restart mechanisms in the HO orientation. (E) CMG bypass of RNAP would leave ssDNA exposed, allowing PrimPol recruitment. Primer synthesis by PrimPol allows the fork to continue, leaving behind ssDNA that can be replicated by a gap-filling mechanism once transcription is complete. (F) ssDNA resulting from bypass and repriming or a failure to reprime, and which persists behind the replication fork, could serve as a template for the formation of hybrids. Hybrids could form through de novo synthesis by RNAP (top) or through the association of nascent RNA resulting from transcription after the fork has passed (bottom). (G) Restart of a stalled fork initiated by SLX4 and MUS81-mediated fork cleavage is thought to relieve the torsional stress, resulting in resolution of the R-loop formed during a HO conflict. Religation of the fork by XRCC4/LIG4 and ELL-mediated restart of transcription allows both replication and transcription to continue. Prior to fork cleavage, RAD51 may promote fork reversal. RECQ1 is needed to reset the fork and RECQ5 promotes removal of RAD51.
