Figure 8 |. TAT-EE3 alleviates ischemic stroke by preserving pro-survival signaling.
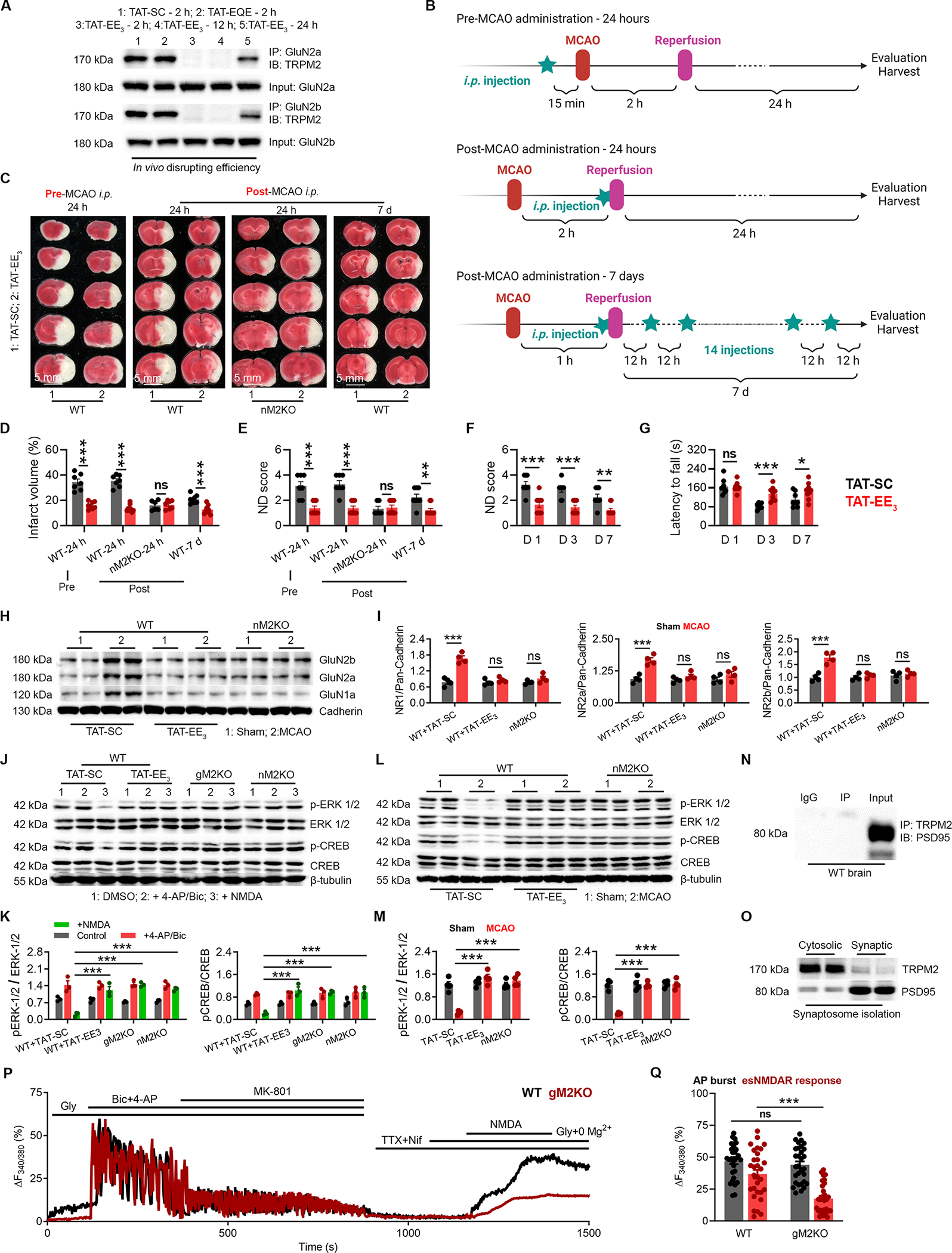
(A) Co-immunoprecipitation (Co-IP) of TRPM2 and GluN2a/2b in the brain lysates from WT mice after MCAO with the treatment of TAT-SC for 2 h, TAT-EQE for 2 h, and TAT-EE3 for 2 h, 12 h and 24 h. IP using anti-GluN2a/2b and IB using anti-TRPM2.
(B) Graphic illustration of injection strategy. For pre-MCAO administration, TAT-EE3 was injected intraperitoneally (i.p.) 30 min before the MCAO. For post-MCAO administration, TAT-EE3 was injected right before the reopening of the occulated MCA to best mimic the treatment of ischemic stroke under clinical situation. For examining the long-term protective effects, mice were subjected to a 1-h MCAO, and TAT-EE3 was administered every 12 hours based on the pre-evaluated in vivo disrupting efficacy (Fig 8A).
(C-G), TAT-EE3 protects WT mice against MCAO. (C), TTC staining of brain slices. (D), Mean infarct volume. (E), Average neurological deficit (ND) score. Average ND score (F) and average latency to fall time (G) for the long-term MCAO experiment (Neuron-specific TRPM2 knockout (nM2KO)).
(H-I), WB analysis of the surface expression of NMDARs in the brain (n=4/group).
(J-K), WBof ERK1/2 and CREB phosphorylation in cultured neurons isolated from global TRPM2 knockout (gM2KO) and nM2KO mice, and WT littermates. WT neurons were pre-treated with TAT-EE3 or TAT-SC overnight (n=3/group).
(L-M), WB of ERK1/2 and CREB phosphorylation in the brains from WT and nM2KO mice subjected to MCAO (n=4/group).
(N) IP using anti-PSD-95 and IB using anti-TRPM2.
(O) WB of TRPM2 expression in synaptosomes.
(P-Q) Isolation of extrasynaptic NMDAR-mediated Ca2+ response in WT and gM2KO neurons. (P) Representative Ca2+ imaging traces (Q) Quantification of AP burst (synaptic NMDAR-mediated response) induced by 4-AP/Bic, and extrasynaptic NMDAR-mediated response by NDMA (n=30/group).
(ns, no statistical significance, *, p < 0.05, **, p < 0.01, ***, p < 0.001; ANOVA, Bonferroni’s test; mean ± SEM).
