Highlights
-
•
Primary tumor volume and hypoxic volume has previously not been convincingly related.
-
•
367 patients with head and neck squamous cell carcinoma from 21 different studies using hypoxia-PET
-
•
The hypoxic volume increased significantly with primary tumor volume.
-
•
In larger tumor the hypoxic fraction was significantly higher than in smaller tumors.
Keywords: Head and neck cancer, Hypoxia, PET-imaging, Tumor volume, Individualized radiation therapy
Abstract
Background
Hypoxia and large tumor volumes are negative prognostic factors for patients with head and neck squamous cell carcinoma (HNSCC) treated with radiation therapy (RT). PET-scanning with specific hypoxia-tracers (hypoxia-PET) can be used to non-invasively assess hypoxic tumor volume. Primary tumor volume is readily available for patients undergoing RT. However, the relationship between hypoxic volume and primary tumor volume is yet an open question. The current study investigates the hypotheses that larger tumors contain both a larger hypoxic volume and a higher hypoxic fraction.
Methods
PubMed and Embase were systematically searched to identify articles fulfilling the predefined criteria. Individual tumor data (primary tumor volume and hypoxic volume/fraction) was extracted. Relationship between hypoxic volume and primary tumor volume was investigated by linear regression. The correlation between hypoxic fraction and log2(primary tumor volume) was determined for each cohort and in a pooled analysis individual regression slopes and coefficients of determination (R2) were weighted according to cohort size.
Results
21 relevant articles were identified and individual data from 367 patients was extracted, out of which 323 patients from 17 studies had quantifiable volumes of interest. A correlation between primary tumor volume and PET-determined hypoxic volume was found (P <.001, R2 = 0.46). Larger tumors had a significantly higher fraction of hypoxia compared with smaller tumors (P<.01). The weighted analysis of all studies revealed that for each doubling of the tumor volume, the hypoxic fraction increased by four percentage points.
Conclusion
This study shows correlations between primary tumor volume and hypoxic volume as well as primary tumor volume and the hypoxic fraction in patients with HNSCC. The findings suggest that not only do large tumors contain more cancer cells, they also have a higher proportion of potentially radioresistant hypoxic cells. This knowledge can be important when individualizing RT.
Introduction
Head and neck malignancies include cancer of the lips, oral cavity, larynx, pharynx, paranasal sinuses, the salivary glands and head and neck cancer of unknown primary. Globally, this group constitutes 4% of all cancers with an incidence of 930 000 cases every year with 80–90% of the tumors being squamous cell carcinomas (HNSCC) [1]. Radiation therapy (RT) is one of the cornerstones in the curative setting, with concurrent chemotherapy for high-risk patients [2].
Hypoxia within tumors is a negative prognostic factor associated with poor clinical outcome [3], [4]. Hypoxia is a result of an imbalance between supply of, and demand for oxygen, and is present in about 60% of all head and neck tumors [5], [6]. Methods to determine hypoxia include invasive Eppendorf pO2 histography, through indirect estimations by using RNA sequencing data, and immunohistochemical staining of endogenous (e.g. carbon anhydrase IX, hypoxia-inducible factor-1 α) or exogenous (e.g. pimonidazole) markers [7], [8], [9], [10]. Radiolabeled exogenous markers (tracers) accumulating in hypoxic tissues can be detected by positron emission tomography (hypoxia-PET), providing a non-invasive method to assess intratumor hypoxia [11]. Tracers such as 18F-fluoromisonidazole (FMISO) and 18F-fluoroazomycin-arabinofuranoside (FAZA) have been shown to be prognostic for patients with HNSCC treated with RT [12]. Hypoxia-PET is often combined with computed tomography (CT) enabling assessment of both the hypoxic volume and the primary tumor volume.
A large primary tumor volume is also a well-known negative prognostic factor for patients with HNSCC [13], [14], [15], [16]. However, the relationship between the hypoxic volume and primary tumor volume has been addressed without any conclusive findings. Using Eppendorf histography, Dunst et al. found a strong correlation between total tumor volume and hypoxic volume, and emphasized a need for newer and more precise methods for measuring hypoxic volume [17]. Chatterjee et al. used hypoxia-PET and showed a correlation between tumor volume and hypoxic tumor volume in a cohort of 18 HNSCC patients [18]. Contrary, Höckel et al. could not show any correlation between tumor oxygenation and tumor size when measuring hypoxia with the Eppendorf histography in uterine cervical cancer [19]. Neither did Stadler et al. find a clear correlation between hypoxic fraction and tumor volume when examining patients with HNSCC using the Eppendorf histograph [3]. Thereby, the relationship between tumor volume and hypoxic volume in human patients has still not been convincingly established.
The aim of our study was therefore to investigate the relationship between primary tumor volume and the hypoxic volume as well as between primary tumor volume and the hypoxic fraction by extracting and compiling published individual patient data from multiple studies using hypoxia-PET in HNSCC patients. The hypothesis was that larger tumors contain both a larger hypoxic volume and a higher hypoxic fraction.
Material and methods
In this study a scoping review was conducted according to Arksey’s and O’Malley’s methodology from 2005, subsequently clarified and enhanced by Levac, Colquhoun and O’Brien in 2010 [20], [21]. The research question was: “Does larger tumors contain a higher fraction of intratumoral hypoxia?”. The databases PubMed and Embase were searched with the help from a professional librarian using keywords and medical subject headings in combination. Search strategies were related to head and neck cancer, tumor hypoxia and PET-scanning, and a limitation to English language was used (Supplementary Table 1 and 2). The records were imported to the reference management tool Endnote and further on to the screening and data extraction tool Covidence, where duplicates were automatically removed. The remaining titles and abstracts and subsequently full text articles were screened according to the predefined selection criteria (Supplementary Table 3). Several studies gave the appearance of possessing the coveted data for individual patients although not presenting it in the article nor in the supplementary material. In these cases an email to the corresponding address was sent, kindly requesting the individual data. Individual patient data (primary tumor volume/primary gross tumor volume (GTV-T) and hypoxic volume) was extracted from all the relevant articles.
Statistics
Patient data was imported to Microsoft Excel and the hypoxic fraction (defined as the hypoxic volume divided by the tumor volume) for each patient was calculated (if not already provided). Relationship between primary tumor volume and hypoxic volume was determined by linear regression. To increase the possibility of interpretation of the hypoxic fraction, tumor volumes were transformed to the binary logarithms (log2). For each study cohort, hypoxic fraction vs. log2 (primary tumor volume) was plotted and the statistical relationship was determined by linear regression. The individual regression slopes and the coefficients of determination (R2) were weighted according to cohort size. Comparisons between two groups were determined using the Wilcoxon rank-sum test. In addition, a pooled analysis was conducted. To account for the different thresholds and methods used, we separately normalized the hypoxic fractions in all cohorts by defining hypoxic fractionArbitrary Unit = 1 at the median tumor volume. This was made by calculating Δy between the fitted linear regression-line and the arbitrary point y = 1 for tumor volume = 25 cm3 (median tumor volume). Δy was then added to the individual data points (hypoxic fractions), leaving the regression slope unaffected. A linear regression for the normalized hypoxic fractions was then performed. Different threshold for determining hypoxic fraction was investigated by Spearman’s rank correlation coefficient (ρ) for a subset of patients with available data. Statistical calculations were performed in Microsoft Excel and in R (version 3.6.3) using RStudio (version 1.2.5042). P-values < 0.05 were determined statistically significant.
Results
Data acquisition
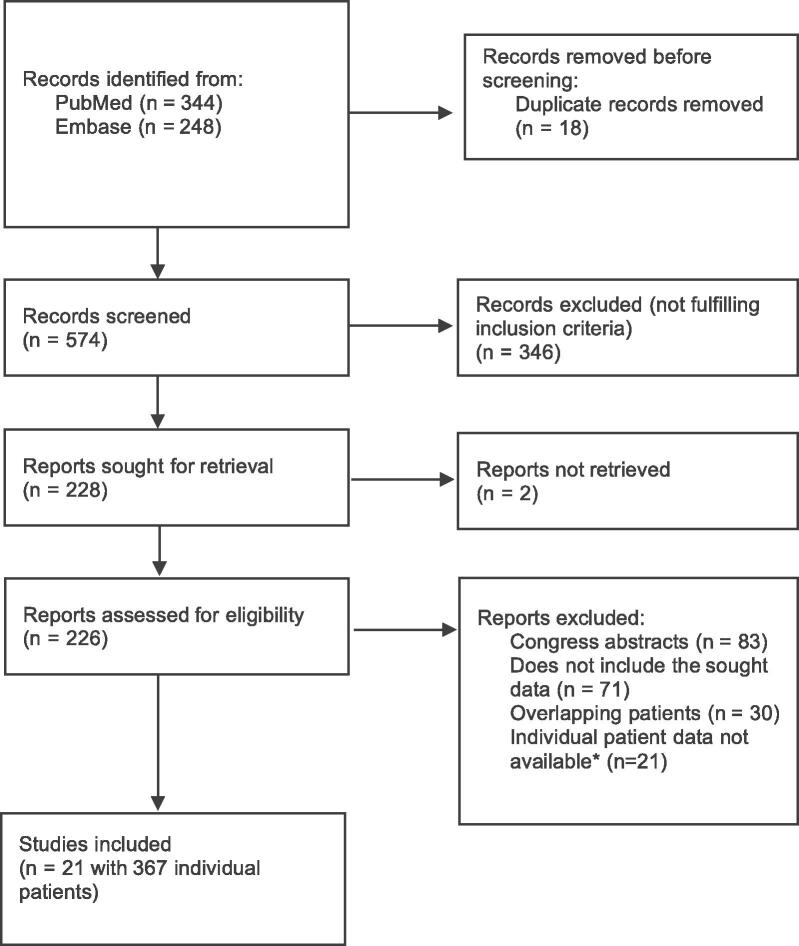
The search in the databases PubMed and Embase resulted in 344 and 248 records respectively which added up to 592 records in total (Fig. 1). Individual data was extracted from 21 articles who met the preset criteria, with a total number of 367 individual patients (Table 1). In three of the patients the hypoxic volume was larger than the primary tumor volume, generating a hypoxic fraction > 1. For these patients the hypoxic fraction was set to 1. In three studies the volumes measured could concern either the primary tumor or lymph nodes, therefore a decision was made to exclude these studies from the pooled analysis. The same was done for a study presenting tumor SUVmax / muscle SUVmean (T/M) instead of hypoxic volume. Finally, 17 studies with a total of 323 individual patients were included in the pooled analysis.
Fig. 1.
Flow chart of the study selection process. * Individual patient data was requested by e-mail to 25 corresponding authors and resulted in 18 non-responders, 3 responders but data not provided, and 4 authors provided data (of which 1 had overlapping data and not included).
Table 1.
Characteristics of the 21 studies.
|
Hypoxic volume vs. tumor volume |
Hypoxic fraction vs. log2(tumor volume) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | # of patients | Tracer used | Cut-off | Range GTVT (min–max) | Range hypoxic fraction | Linear regression coefficient | P-value | R2 | Linear regression coefficient | P-value | R2 | REF | |
| Silvoniemi, A | 2018 | 10 | EF5 | TMR threshold 1.5 | 8.9–73.5 | 0.01–0.43 | 0.403 | 0.002 | 0.70 | 0.0615 | 0.21 | 0.19 | [43] | |
| Mönnich, D | 2017 | 21 | FMISO | TMR threshold 1.4 | 15.9–209.3 | 0–0.71 | 0.104 | 0.09 | 0.10 | −0.0327 | 0.54 | 0.020 | [44] | |
| Kazmierska, J | 2020 | 35 | FMISO | TMR treshold 1.6 | 0.2–174.3 | 0–0.14 | 0.043 | <0.001 | 0.27 | 0.0075 | 0.022 | 0.15 | [45] | |
| Löck,S | 2019 | 42 | FMISO | TBR treshold 1.6 | 5.06–177.85 | 0–1 | 0.307 | <0.001 | 0.36 | 0.0726 | 0.056 | 0.09 | [46] | |
| Zegers, C | 2016 | 20 | HX4 | TMR treshold 1.4 | 2.4–46.6 | 0–0.32 | 0.132 | 0.008 | 0.29 | 0.0377 | 0.049 | 0.20 | [47] | |
| Bollineni, V. R | 2014 | 6 | FAZA | TBR treshold 1.4 | 26–50 | 0.05–0.85 | 1.320 | 0.049 | 0.58 | 0.6382 | 0.068 | 0.61 | [48] | |
| Chang, J | 2013 | 8 | FMISO | TMR treshold 1.5 | 14.5–52.4 | 0.05–0.16 | 0.088 | 0.027 | 0.52 | −0.0142 | 0.56 | 0.06 | [49] | |
| Grosu, A | 2007 | 18 | FAZA | TMR threshold 1.5 | 18.8–115 | 0–0.51 | 0.290 | 0.007 | 0.34 | 0.0541 | 0.12 | 0.14 | [50] | |
| Komar, G | 2014 | 22 | EF5 | TMR of 1.5 | 0.98–45 | 0–0.998 | 0.089 | <0.001 | 0.72 | 0.1053 | 0.026 | 0.22 | [51] | |
| Lehtiö, K | 2004 | 19 | FETNIM | N/A | 1.4–401.6 | 0.095–0.64 | 0.659 | <0.001 | 0.95 | 0.037 | 0.14 | 0.13 | [52] | |
| Lin, Z | 2008 | 7 | FMISO | TBR threshold 1.3 | 23.45–140.8 | 0.03–0.48 | 0.417 | 0.21 | 0.15 | 0.0429 | 0.52 | 0.09 | [53] | |
| Saksø, M | 2020 | 40 | FAZA | TMR threshold 1.6 | 1.6–144.6 | 0–0.84 | 0.086 | <0.001 | 0.37 | 0.0151 | 0.45 | 0.02 | [54] | |
| Servagi-Vernat, S | 2015 | 12 | FAZA | TMR ratio 1.6 | 2.4–73 | 0–0.54 | 0.497 | <0.001 | 0.72 | 0.0873 | 0.021 | 0.43 | [55] | |
| Simoncic, U | 2017 | 6 | FMISO | TMR threshold 1.4 | 11.6–48.5 | 0.01–0.72 | 0.418 | 0.22 | 0.17 | −0.133 | 0.48 | 0.13 | [56] | |
| Bittner, M | 2016 | 16 | FMISO | TMR threshold 1.4 | 9–99 | 0.05–0.99 | 0.754 | <0.001 | 0.88 | 0.0068 | 0.94 | 0.004 | [57] | |
| Nehmeh, S | 2021 | 18 | FMISO | TBR treshold 1.2 | 3.9–34.9 | 0.003–1 | 0.657 | <0.001 | 0.74 | 0.1164 | 0.28 | 0.07 | [58] | |
| Sato, J |
2018 |
23 |
FMISO |
TMR threshold 1.25 |
0.3–36.1 |
0–1 |
0.153 |
<0.001 |
0.58 |
−0.0229 |
0.37 |
0.04 |
[59] | |
| 323 | 0.27§ | <0.001§ | 0.46§ | 0.045† | <0.001* | 0.12† | ||||||||
| Reason for exclusion | ||||||||||||||
| Dirix, P | 2009 | 12 | FMISO | TBR treshold 1.2 | 14.5–85 | 0–0.46 | 0.095 | 0.17 | 0.09 | −0.0307 | 0.603 | 0.028 | [60] | lymph nodes or primary tumor volume |
| Henriques de Figueiredo | 2015 | 10 | FMISO | See publication | 306–518** | 0.01–0.14 | 0.07 | 0.31 | 0.02 | 0.0048 | 0.926 | 0.0011 | [61] | lymph nodes or primary tumor volume |
| Zegers, C | 2015 | 7 | HX4 | TBR treshold 1.2 | 2.6–79.9 | 0–0.15 | 0.05 | 0.01 | 0.70 | 0.0053 | 0.739 | 0.0241 | [62] | lymph nodes or primary tumor volume |
| Minagawa, Y | 2011 | 15 | 62Cu-ATSM | 1.2–76.5 | 1.61–10.94*** | 1.9564 | 0.168 | 0.1406 | [63] | No volume available | ||||
Original and analyzed data for the 21 included studies. For each study cohort, the statistical relationship between primary tumor volume and hypoxic volume (light grey columns) as well as between primary tumor volume (binary logarithm) and hypoxic fraction (dark grey columns) were determined. The bottom four studies were excluded from the pooled analysis. GTVT: primary tumor volume, TMR: tumor-to-muscle ratio, TBR: tumor-to-blood ratio.
* Result of the pooled analysis of normalized data according to Fig. 2B.
** Values representing clinical target volume (CTV) instead of GTVT.
*** (T/M) tumor SUVmax/muscle SUVmean.
Result of the pooled analysis.
Sum of individual analyses, weighted according to cohort size.
Relationship between primary tumor volume and hypoxic volume
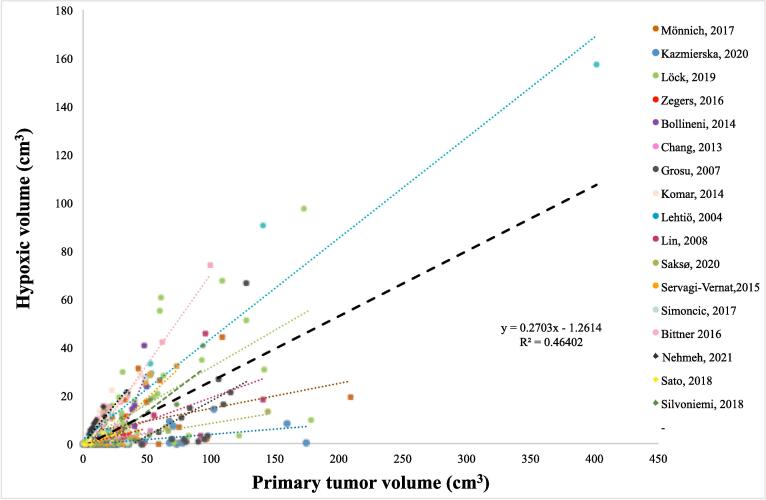
Hypoxic tumor volumes as determined by hypoxia-PET was correlated to primary tumor volume (Fig. 2A). Using linear regression, a significant positive correlation between hypoxia-PET determined hypoxic volume and primary tumor volume for the 323 included patients was found (P <.001, R2 = 0.46). Within the individual studies, significant correlations were found for 15 out of 17 studies (Table 1). Sensitivity analyses by dichotomizing the cohort at different primary tumor volumes and separately analyzing smaller and larger tumors for correlations between primary tumor volume and hypoxic volume revealed similar and significant results throughout (e.g. for primary tumors smaller or larger than 10 cm3 the regression slope was 0.29 and 0.28, respectively with P <.001 in both subgroups).
Fig. 2.
A) The hypoxic volume determined by hypoxia-PET as a function of tumor volume for the 17 cohorts included in the pooled analysis. Every cohort is presented in a unique color, and each point represents one individual patient. Lines denote the linear regression of each cohort and thick black dashed line illustrates the linear regression of 323 patients (with regression coefficients in the figure, P <.001).
Relationship between primary tumor volume and hypoxic fraction
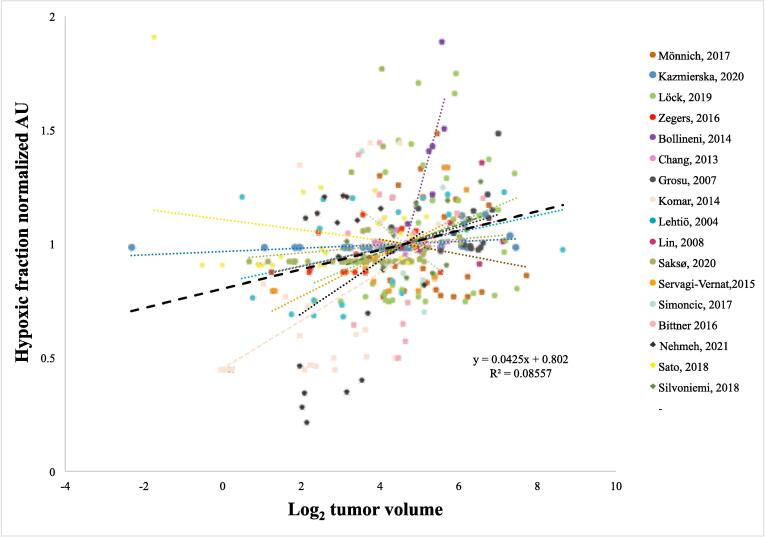
We next investigated the hypoxic fraction in relationship to primary tumor volume. Four of the 17 individual studies showed a positive significant correlation between primary tumor volume and hypoxic fraction (Table 1 and Supplementary Figure S1). In the weighted analysis of all studies a positive correlation between hypoxic fraction and primary tumor volume was found. For each doubling of the primary tumor volume, the hypoxic fraction increased by an average of four percentage points, with a weighted R2-value of 0.12. The median hypoxic fraction for patients with a primary tumor volume less than median was 6% (interquartile range [IQR] 0–25) compared with 13% (IQR 2–39) for patients with a primary tumor volume equal to or larger than the median tumor volume (P <.01). Moreover, in the pooled analysis of normalized hypoxic fractions, a correlation between hypoxic fraction and primary tumor volume was found with a positive slope of 0.042 (95% confidence interval 0.027–0.058, P <.001), corresponding to an average increase of the hypoxic fraction by four percentage points for each primary tumor volume doubling (Fig. 2B). Likewise, the non-normalized pooled analysis showed a positive relationship between hypoxic fraction and primary tumor volume (Supplementary Fig S2). In a subset of 114 patients the correlation between hypoxic fraction and tumor volume was investigated at different thresholds for defining hypoxia (i.e. tumor-to-muscle ratio 1.4–2.0). Significant correlations were found for all hypoxia-defining thresholds, with a tendency towards stronger correlations for higher thresholds (Table 2).
Table 2.
Correlation between hypoxic fraction and tumor volume for different thresholds to define hypoxia. Values in subscript (1.4-2.0) denote the tumor-to-muscle ratio (TMR) for defining hypoxia. Data for 114 patients from [12] including the data from [44], [46], [55] and 39 additional patients.
| Spearman correlation | P-value | |
|---|---|---|
| Hypoxic fraction1.4 | 0.34 | <0.001 |
| Hypoxic fraction1.6 | 0.37 | <0.001 |
| Hypoxic fraction1.8 | 0.38 | <0.001 |
| Hypoxic fraction2.0 | 0.42 | <0.001 |
Discussion
In this pooled analyses of 323 HNSCC patients from 17 different studies using hypoxia-PET we have found a correlation between the primary tumor volume and the hypoxic volume. Moreover, the hypoxic fraction correlated with the primary tumor volume, and increased significantly with increasing primary tumor volume. Thereby, patients who present with a large tumor have, in general, both a larger volume of hypoxic cells as well as a higher proportion of hypoxic cells compared to patients with a smaller tumor.
The present results add substantial knowledge to earlier diverging findings. Similar to the current findings, positive correlations between tumor volume and hypoxic volumes have been found using hypoxia-PET and for Eppendorf histography [17], [18], [22]. In contrary, Saksø M. et al. did not observe any correlation between hypoxia and tumor volume in human head and neck cancer when using both Eppendorf histography and FMISO-PET [23]. Important to note is that their measurements were made in single lymph nodes rather than in primary tumors, which may have affected the result. Neither did Stadler et al. find a clear correlation between tumor volume and hypoxic fraction using Eppendorf histography [3]. These measurements were performed either in the primary tumor or a lymph node metastasis for each patient, which potentially could have affected the outcome. Vaupel et al. did not observe any correlation between the occurrence of hypoxia and tumor size (comparing T1-2 with T3-4 tumors) when using Eppendorf histography in breast cancer tumors, however these observations were only made in a small cohort of 15 patients [24].
A relationship between tumor volume and hypoxia was demonstrated in animal models already in the 60/70′s [25], [26], [27], although the presence of tumor hypoxia and the relationship to tumor volume may be cell line dependent [28], [29]. For six different squamous cell carcinoma models, no relationship between tumor volume and hypoxic fraction was found when hypoxia was assessed after ten fractions of RT [30]. The current study suggests a relationship between tumor volume and tumor hypoxia before start of RT in human HNSCC. Considering that hypoxic tumor cells are more radioresistant, an increased number of hypoxic tumor cells is likely to contribute to the poorer outcome seen in larger tumors. Our results suggest that when a tumor doubles in size, the hypoxic fraction increases by four percentage points.
Several imaging parameters are obtained through hypoxia-PET and consensus for its use is lacking. Cut-off values for defining hypoxic volumes (e.g. 1.4 or 1.6 times the background level in muscle) and normalization methods (e.g. comparing to background level in blood, muscle or cerebellum) differ between studies. Parameters such as SUVmax (the maximum of the Standardized-Uptake-Value) and presence of tumor-to-muscle ratio > 2 have proven informative [12], [22]. Image acquisition is typically obtained through static scans, but dynamic scans can provide additional information of perfusion and tracer retention [31]. Hypoxia-PET measured after 1–2 weeks of RT could be more informative compared with before RT [22], [32]. In addition, several tracers with different characteristics are available. Thereby, the optimal usage of hypoxia-PET in HNSCC is yet to be defined. Encouragingly, the prognostic value of hypoxia-PET was recently confirmed in a large meta-analysis, and the two most commonly used tracers (FMISO and FAZA) were found to provide equivalent results [12]. One of the advantages of using hypoxia-PET is its ability to non-invasively visualize hypoxic volumes, enabling direct comparisons with CT-determined tumor volume. Eppendorf histography has been extensively used in the past and can be sampled through tumors, thus providing spatial distribution of hypoxia, but requires an invasive procedure [17], [24]. RNA-sequencing using hypoxic profiles can be used to identify hypoxic tumors and have been shown to be prognostic for patients with HNSCC treated with RT [33], [34]. However, no spatial information is provided. Interestingly, an interaction between CT-determined tumor volume and RNA-seq-based hypoxic profiles was described by Linge et al., and the gene profile was only prognostic for patients with small tumors [33].
There are a number of limitations in the current study. First, being retrospective, this study naturally has a lower degree of evidence. We strived to minimize the risk for selection bias by using beforehand defined inclusion- and exclusion criteria. The cohorts used different tracers and assessment methods leading to heterogeneity in the pooled analysis. Despite this heterogeneity, a statistically highly significant relationship was found in the pooled analysis. Moreover, the study focuses on hypoxia in primary tumors and relies on the volumes as identified in the original target delineations. Hypoxia-PET assessments may underestimate hypoxia in small tumors, which would affect the current analyses [35]. We therefore conducted the sensitivity analyses described above, with similar correlations for small and large tumors. Inherent disabilities, such as partial volume effects, for detecting hypoxia with hypoxia-PET in smaller tumors might still be present and would then contribute to inflate the current results [36]. It has previously been shown that human papillomavirus (HPV)-positive cell lines respond differently to hypoxia [37], and a recent clinical trial investigated the use hypoxia-PET to de-escalate RT for patients with HPV-positive oropharyngeal HNSCC [38]. We could, however, not investigate the subgroup of HPV-associated HNSCC since HPV-status was only available for a small subset of patients. It should be noted that the term hypoxia refers to features identified by the specific hypoxia-PET tracers and are therefore only surrogate markers of the underlying hypoxia The analyses do not investigate any relationships to clinical outcome after RT. Lastly, the results refer to hypoxia before start of RT, and changes during RT or by physiological changes over time are outside the scope of the current study, and it could be noted that hypoxia measured during RT might provide more prognostication than baseline hypoxia [22], [32].
Looking into the future, our result may be useful for individualizing RT in HNSCC-patients. Since hypoxia refers radioresistance, treatment intensification such as dose-escalation or addition of hypoxia-sensitizing agents could be indicated for patients with larger tumors. We have previously described that intensified RT was more beneficial in patients with large tumors [16], [39]. Similar results were found in other HNSCC and lung cancer trials [40], [41], [42]. Our present results could suggest that the underlying mechanism for the increased efficacy of intensified RT in large tumors might be related to tumor hypoxia. In addition, since hypoxia-PET is relatively expensive, time consuming and associated with potential patient discomfort due to long imaging and post acquisition times, primary tumor volume might be used as a screening method for further hypoxia imaging.
Conclusion
This study shows significant positive correlations between primary tumor volume and both hypoxic volume as well as hypoxic fraction in human head and neck cancers. The findings suggest that not only do large tumors contain more cells, they also have a higher proportion of potentially radioresistant hypoxic cells. This knowledge may be of value in the development of a more individualized radiation therapy. Our findings will need to be validated in another cohort and by using other methods to assess tumor hypoxia.
Funding
Governmental research funding (Yngre ALF). Mrs Berta Kamprad Foundation (grant no FBKS 2020–19-301).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
We would like to show our gratitude to PhD Catharina Zegers (Dept. of Radiation Oncology, Maastricht, Netherlands), Prof. Dr. Steffen Löck (OncoRay Center, Dresden, Germany) and Dr. Joanna Kazmierska (Radiotherapy Dept. II in Greater Poland Cancer Center, Poznan, Poland) who all answered our emails and provided us with individual data from their studies. We would also like to thank the library of the Medical Faculty of Lund University for their valuable expertise.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.06.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., et al. International Agency for Research on Cancer; Lyon, France: 2018. Global Cancer Observatory: Cancer Today.https://gco.iarc.fr/today (accessed February 25, 2022) [Google Scholar]
- 2.Machiels J.-P., René Leemans C., Golusinski W., Grau C., Licitra L., Gregoire V. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS–ESMO–ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1462–1475. doi: 10.1016/j.annonc.2020.07.011. [DOI] [PubMed] [Google Scholar]
- 3.Stadler P., Becker A., Jürgen Feldmann H., Hänsgen G., Dunst J., Würschmidt F., et al. Influence of the hypoxic subvolume on the survival of patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 1999;44(4):749–754. doi: 10.1016/s0360-3016(99)00115-7. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard J. Hypoxic radiosensitization: adored and ignored. J Clin Oncol. 2007;25:4066–4074. doi: 10.1200/JCO.2007.12.7878. [DOI] [PubMed] [Google Scholar]
- 5.Chirla R., Marcu L.G. PET-based quantification of statistical properties of hypoxic tumor subvolumes in head and neck cancer. Phys Medica. 2016;32:23–35. doi: 10.1016/j.ejmp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Mortensen L.S., Johansen J., Kallehauge J., Primdahl H., Busk M., Lassen P., et al. FAZA PET/CT hypoxia imaging in patients with squamous cell carcinoma of the head and neck treated with radiotherapy: Results from the DAHANCA 24 trial. Radiother Oncol. 2012;105(1):14–20. doi: 10.1016/j.radonc.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Rudat V., Stadler P., Becker A., Vanselow B., Dietz A., Wannenmacher M., et al. Predictive value of the tumor oxygenation by means of pO2 histography in patients with advanced head and neck cancer. Strahlenther Onkol. 2001;177(9):462–468. doi: 10.1007/pl00002427. [DOI] [PubMed] [Google Scholar]
- 8.Eustace A., Mani N., Span P.N., Irlam J.J., Taylor J., Betts G.N.J., et al. A 26-Gene Hypoxia Signature Predicts Benefit from Hypoxia-Modifying Therapy in Laryngeal Cancer but Not Bladder Cancer. Clin Cancer Res. 2013;19:4879–4888. doi: 10.1158/1078-0432.CCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rademakers S.E., Hoogsteen I.J., Rijken P.F., Oosterwijk E., Terhaard C.H., Doornaert P.A., et al. Pattern of CAIX expression is prognostic for outcome and predicts response to ARCON in patients with laryngeal cancer treated in a phase III randomized trial. Radiother Oncol. 2013;108(3):517–522. doi: 10.1016/j.radonc.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 10.Hoogsteen I.J., Lok J., Marres H.A.M., Takes R.P., Rijken P.F.J.W., van der Kogel A.J., et al. Hypoxia in larynx carcinomas assessed by pimonidazole binding and the value of CA-IX and vascularity as surrogate markers of hypoxia. Eur J Cancer. 2009;45(16):2906–2914. doi: 10.1016/j.ejca.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Fleming I.N., Manavaki R., Blower P.J., West C., Williams K.J., Harris A.L., et al. Imaging tumour hypoxia with positron emission tomography. Br J Cancer. 2015;112(2):238–250. doi: 10.1038/bjc.2014.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zschaeck S., Löck S., Hofheinz F., Zips D., Saksø Mortensen L., Zöphel K., et al. Individual patient data meta-analysis of FMISO and FAZA hypoxia PET scans from head and neck cancer patients undergoing definitive radio-chemotherapy. Radiother Oncol. 2020;149:189–196. doi: 10.1016/j.radonc.2020.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Lok B.H., Setton J., Caria N., Romanyshyn J., Wolden S.L., Zelefsky M.J., et al. Intensity-modulated radiation therapy in oropharyngeal carcinoma: Effect of tumor volume on clinical outcomes. Int J Radiat Oncol Biol Phys. 2012;82(5):1851–1857. doi: 10.1016/j.ijrobp.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao K.S.C., Ozyigit G., Blanco A.I., Thorstad W.L., Deasy J.O., Haughey B.H., et al. Intensity-modulated radiation therapy for oropharyngeal carcinoma: Impact of tumor volume. Int J Radiat Oncol Biol Phys. 2004;59(1):43–50. doi: 10.1016/j.ijrobp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Rutkowski T. The role of tumor volume in radiotherapy of patients with head and neck cancer. Radiat Oncol. 2014;9:1–9. doi: 10.1186/1748-717X-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adrian G., Carlsson H., Kjellén E., Sjövall J., Zackrisson B., Nilsson P., et al. Primary tumor volume and prognosis for patients with p16-positive and p16-negative oropharyngeal squamous cell carcinoma treated with radiation therapy. Radiat Oncol. 2022;17(1) doi: 10.1186/s13014-022-02074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunst J., Stadler P., Becker A., Lautenschläger C., Pelz T., Hänsgen G., et al. Tumor volume and tumor hypoxia in head and neck cancers: The amount of the hypoxic volume is importantTumorvolumen und Hypoxie in Kopf-Hals-Tumoren. Das absolute hypoxische Volumen ist entscheidend. Strahlentherapie Und Onkol. 2003;179(8):521–526. doi: 10.1007/s00066-003-1066-4. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee A., Gupta T., Rangarajan V., Purandare N., Kunder S., Murthy V., et al. Optimal timing of fluorine-18-fluoromisonidazole positron emission tomography/computed tomography for assessment of tumor hypoxia in patients with head and neck squamous cell carcinoma. Nucl Med Commun. 2018;39(9):859–864. doi: 10.1097/MNM.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 19.Hockel M., Schlenger K., Aral B., Mitze M., Schaffer U., Vaupel P. Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res. 1996;56:4509–4515. [PubMed] [Google Scholar]
- 20.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arksey H., O’Malley L. Scoping studies: Towards a methodological framework. Int J Soc Res Methodol Theory Pract. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 22.Zips D., Zöphel K., Abolmaali N., Perrin R., Abramyuk A., Haase R., et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol. 2012;105(1):21–28. doi: 10.1016/j.radonc.2012.08.019. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen L.S., Buus S., Nordsmark M., Bentzen L., Munk O.L., Keiding S., et al. Identifying hypoxia in human tumors: A correlation study between 18F-FMISO PET and the Eppendorf oxygen-sensitive electrode. Acta Oncol (Madr) 2010;49(7):934–940. doi: 10.3109/0284186X.2010.516274. [DOI] [PubMed] [Google Scholar]
- 24.Vaupel P., Schlenger K., Knoop C., Höckel M. Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res. 1991;51:3316–3322. [PubMed] [Google Scholar]
- 25.Belli J.A., Andrews J.R. Relationship Between Tumor Growth and Radiosensitivity. JNCI J Natl Cancer Inst. 1963;31:689–703. doi: 10.1093/jnci/31.3.689. [DOI] [PubMed] [Google Scholar]
- 26.Shipley W.U., Stanley J.A., Steel G.G. Tumor size dependency in the radiation response of the Lewis lung carcinoma. Cancer Res. 1975;35:2488–2493. [PubMed] [Google Scholar]
- 27.Stanley J.A., Shipley W.U., Steel G.G. Influence of tumour size on hypoxic fraction and therapeutic sensitivity of Lewis lung tumour. Br J Cancer. 1977;36(1):105–113. doi: 10.1038/bjc.1977.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shibamoto Y., Yukawa Y., Tsutsui K., Takahashi M., Abe M. Variation in the hypoxic fraction among mouse tumors of different types, sizes, and sites. Jpn J Cancer Res. 1986;77:908–915. [PubMed] [Google Scholar]
- 29.Gerweck L.E., Zaidi S.T., Zietman A. Multivariate determinants of radiocurability. I: Prediction of single fraction tumor control doses. Int J Radiat Oncol Biol Phys. 1994;29:57–66. doi: 10.1016/0360-3016(94)90226-7. [DOI] [PubMed] [Google Scholar]
- 30.Yaromina A., Kroeber T., Meinzer A., Boeke S., Thames H., Baumann M., et al. Exploratory study of the prognostic value of microenvironmental parameters during fractionated irradiation in human squamous cell carcinoma xenografts. Int J Radiat Oncol Biol Phys. 2011;80(4):1205–1213. doi: 10.1016/j.ijrobp.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Welz S., Mönnich D., Pfannenberg C., Nikolaou K., Reimold M., La Fougère C., et al. Prognostic value of dynamic hypoxia PET in head and neck cancer: Results from a planned interim analysis of a randomized phase II hypoxia-image guided dose escalation trial. Radiother Oncol. 2017;124(3):526–532. doi: 10.1016/j.radonc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Löck S., Perrin R., Seidlitz A., Bandurska-Luque A., Zschaeck S., Zöphel K., et al. Residual tumour hypoxia in head-and-neck cancer patients undergoing primary radiochemotherapy, final results of a prospective trial on repeat FMISO-PET imaging. Radiother Oncol. 2017;124(3):533–540. doi: 10.1016/j.radonc.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 33.Linge A., Lohaus F., Löck S., Nowak A., Gudziol V., Valentini C., et al. HPV status, cancer stem cell marker expression, hypoxia gene signatures and tumour volume identify good prognosis subgroups in patients with HNSCC after primary radiochemotherapy: A multicentre retrospective study of the German Cancer Consortium Radiation. Radiother Oncol. 2016;121:364–373. doi: 10.1016/j.radonc.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 34.van der Heijden M., Essers P.B.M., de Jong M.C., de Roest R.H., Sanduleanu S., Verhagen C.V.M., et al. Biological Determinants of Chemo-Radiotherapy Response in HPV-Negative Head and Neck Cancer: A Multicentric External Validation. Front Oncol. 2020;9 doi: 10.3389/fonc.2019.01470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bandurska-Luque A., Löck S., Haase R., Richter C., Zöphel K., Perrin R., et al. Correlation between FMISO-PET based hypoxia in the primary tumour and in lymph node metastases in locally advanced HNSCC patients. Clin Transl Radiat Oncol. 2019;15:108–112. doi: 10.1016/j.ctro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelada O.J., Carlson D.J. Molecular Imaging of Tumor Hypoxia with Positron Emission Tomography. Radiat Res. 2014;181:335–349. doi: 10.1667/RR13590.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Göttgens E.-L., Bussink J., Ansems M., Hammond E.M., Span P.N. AKT inhibition as a strategy for targeting hypoxic HPV-positive HNSCC. Radiother Oncol. 2020;149:1–7. doi: 10.1016/j.radonc.2020.04.048. [DOI] [PubMed] [Google Scholar]
- 38.Riaz N., Sherman E., Pei X., Schöder H., Grkovski M., Paudyal R., et al. Precision Radiotherapy: Reduction in Radiation for Oropharyngeal Cancer in the 30 ROC Trial. J Natl Cancer Inst. 2021;113(6):742–751. doi: 10.1093/jnci/djaa184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adrian G., Gebre-Medhin M., Kjellén E., Wieslander E., Zackrisson B., Nilsson P. Altered fractionation diminishes importance of tumor volume in oropharyngeal cancer: Subgroup analysis of ARTSCAN-trial. Head Neck. 2020;42:2099–2105. doi: 10.1002/hed.26142. [DOI] [PubMed] [Google Scholar]
- 40.Horiot J.C., Le Fur R., N'Guyen T., Chenal C., Schraub S., Alfonsi S., et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol. 1992;25(4):231–241. doi: 10.1016/0167-8140(92)90242-m. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L., West B.T., Hayman J.A., Lyons S., Cease K., Kong F.M., et al. High Radiation Dose May Reduce the Negative Effect of Large Gross Tumor Volume in Patients With Medically Inoperable Early-Stage Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2007;68:103–110. doi: 10.1016/j.ijrobp.2006.11.051. [DOI] [PubMed] [Google Scholar]
- 42.Soliman M., Yaromina A., Appold S., Zips D., Reiffenstuhl C., Schreiber A., et al. GTV differentially impacts locoregional control of non-small cell lung cancer (NSCLC) after different fractionation schedules: Subgroup analysis of the prospective randomized CHARTWEL trial. Radiother Oncol. 2013;106(3):299–304. doi: 10.1016/j.radonc.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Silvoniemi A., Suilamo S., Laitinen T., Forsback S., Löyttyniemi E., Vaittinen S., et al. Repeatability of tumour hypoxia imaging using [18F]EF5 PET/CT in head and neck cancer. Eur J Nucl Med Mol Imaging. 2018;45(2):161–169. doi: 10.1007/s00259-017-3857-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mönnich D., Thorwarth D., Leibfarth S., Pfannenberg C., Reischl G., Mauz P.-S., et al. Overlap of highly FDG-avid and FMISO hypoxic tumor subvolumes in patients with head and neck cancer. Acta Oncol (Madr) 2017;56(11):1577–1582. doi: 10.1080/0284186X.2017.1363910. [DOI] [PubMed] [Google Scholar]
- 45.Kazmierska J., Cholewinski W., Piotrowski T., Sowinska A., Bak B., Cegła P., et al. Assessment of tumour hypoxia, proliferation and glucose metabolism in head and neck cancer before and during treatment. Br J Radiol. 2020;93(1106):20180781. doi: 10.1259/bjr.20180781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Löck S., Linge A., Seidlitz A., Bandurska-Luque A., Nowak A., Gudziol V., et al. Repeat FMISO-PET imaging weakly correlates with hypoxia-associated gene expressions for locally advanced HNSCC treated by primary radiochemotherapy. Radiother Oncol. 2019;135:43–50. doi: 10.1016/j.radonc.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 47.Zegers C.M.L., Hoebers F.J.P., van Elmpt W., Bons J.A., Öllers M.C., Troost E.G.C., et al. Evaluation of tumour hypoxia during radiotherapy using [(18)F]HX4 PET imaging and blood biomarkers in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2016;43(12):2139–2146. doi: 10.1007/s00259-016-3429-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bollineni V.R., Koole M.J.B., Pruim J., Brouwer C.L., Wiegman E.M., Groen H.J.M., et al. Dynamics of tumor hypoxia assessed by 18F-FAZA PET/CT in head and neck and lung cancer patients during chemoradiation: Possible implications for radiotherapy treatment planning strategies. Radiother Oncol. 2014;113(2):198–203. doi: 10.1016/j.radonc.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 49.Chang J.H., Wada M., Anderson N.J., Lim Joon D., Lee S.T., Gong S.J., et al. Hypoxia-targeted radiotherapy dose painting for head and neck cancer using 18F-FMISO PET: A biological modeling study. Acta Oncol (Madr) 2013;52(8):1723–1729. doi: 10.3109/0284186X.2012.759273. [DOI] [PubMed] [Google Scholar]
- 50.Grosu A.-L., Souvatzoglou M., Röper B., Dobritz M., Wiedenmann N., Jacob V., et al. Hypoxia Imaging With FAZA-PET and Theoretical Considerations With Regard to Dose Painting for Individualization of Radiotherapy in Patients With Head and Neck Cancer. Int J Radiat Oncol Biol Phys. 2007;69(2):541–551. doi: 10.1016/j.ijrobp.2007.05.079. [DOI] [PubMed] [Google Scholar]
- 51.Komar G., Lehtiö K., Seppänen M., Eskola O., Levola H., Lindholm P., et al. Prognostic value of tumour blood flow, [18F]EF5 and [18F]FDG PET/CT imaging in patients with head and neck cancer treated with radiochemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(11):2042–2050. doi: 10.1007/s00259-014-2818-3. [DOI] [PubMed] [Google Scholar]
- 52.Lehtiö K., Eskola O., Viljanen T., Oikonen V., Grönroos T., Sillanmäki L., et al. Imaging perfusion and hypoxia with PET to predict radiotherapy response in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59(4):971–982. doi: 10.1016/j.ijrobp.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 53.Lin Z., Mechalakos J., Nehmeh S., Schoder H., Lee N., Humm J., et al. The Influence of Changes in Tumor Hypoxia on Dose-Painting Treatment Plans Based on 18F-FMISO Positron Emission Tomography. Int J Radiat Oncol Biol Phys. 2008;70(4):1219–1228. doi: 10.1016/j.ijrobp.2007.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saksø M., Mortensen L.S., Primdahl H., Johansen J., Kallehauge J., Hansen C.R., et al. Influence of FAZA PET hypoxia and HPV-status for the outcome of head and neck squamous cell carcinoma (HNSCC) treated with radiotherapy: Long-term results from the DAHANCA 24 trial ( NCT01017224) Radiother Oncol. 2020;151:126–133. doi: 10.1016/j.radonc.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 55.Servagi-Vernat S., Differding S., Sterpin E., Hanin F.-X., Labar D., Bol A., et al. Hypoxia-guided adaptive radiation dose escalation in head and neck carcinoma: a planning study. Acta Oncol. 2015;54(7):1008–1016. doi: 10.3109/0284186X.2014.990109. [DOI] [PubMed] [Google Scholar]
- 56.Simoncic U., Leibfarth S., Welz S., Schwenzer N., Schmidt H., Reischl G., et al. Comparison of DCE-MRI kinetic parameters and FMISO-PET uptake parameters in head and neck cancer patients. Med Phys. 2017;44(6):2358–2368. doi: 10.1002/mp.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bittner M.-I., Wiedenmann N., Bucher S., Hentschel M., Mix M., Rücker G., et al. Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radiochemotherapy. Acta Oncol (Madr) 2016;55(11):1299–1304. doi: 10.1080/0284186X.2016.1219046. [DOI] [PubMed] [Google Scholar]
- 58.Nehmeh S.A., Moussa M.B., Lee N., Zanzonico P., Gönen M., Humm J.L., et al. Comparison of FDG and FMISO uptakes and distributions in head and neck squamous cell cancer tumors. EJNMMI Res. 2021;11(1) doi: 10.1186/s13550-021-00767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato J., Kitagawa Y., Watanabe S., Asaka T., Ohga N., Hirata K., et al. Hypoxic volume evaluated by 18F-fluoromisonidazole positron emission tomography (FMISO-PET) may be a prognostic factor in patients with oral squamous cell carcinoma: preliminary analyses. Int J Oral Maxillofac Surg. 2018;47(5):553–560. doi: 10.1016/j.ijom.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 60.Dirix P., Vandecaveye V., De Keyzer F., Stroobants S., Hermans R., Nuyts S. Dose painting in radiotherapy for head and neck squamous cell carcinoma: Value of repeated functional imaging with 18F-FDG PET, 18F-fluoromisonidazole PET, diffusion-weighted MRI, and dynamic contrast-enhanced MRI. J Nucl Med. 2009;50:1020–1027. doi: 10.2967/jnumed.109.062638. [DOI] [PubMed] [Google Scholar]
- 61.Henriques de Figueiredo B., Zacharatou C., Galland-Girodet S., Benech J., De Clermont-Gallerande H., Lamare F., et al. Hypoxia imaging with [18F]-FMISO-PET for guided dose escalation with intensity-modulated radiotherapy in head-and-neck cancersHypoxie-Bildgebung mit [18F]-FMISO-PET für geführte Dosissteigerung bei intensitätsmodulierter Strahlentherapie bei Kopf-Hals-Tumoren. Strahlenther Onkol. 2015;191(3):217–224. doi: 10.1007/s00066-014-0752-8. [DOI] [PubMed] [Google Scholar]
- 62.Zegers C.M.L., van Elmpt W., Szardenings K., Kolb H., Waxman A., Subramaniam R.M., et al. Repeatability of hypoxia PET imaging using [18F]HX4 in lung and head and neck cancer patients: a prospective multicenter trial. Eur J Nucl Med Mol Imaging. 2015;42(12):1840–1849. doi: 10.1007/s00259-015-3100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Minagawa Y., Shizukuishi K., Koike I., Horiuchi C., Watanuki K., Hata M., et al. Assessment of tumor hypoxia by 62Cu-ATSM PET/CT as a predictor of response in head and neck cancer: a pilot study. Ann Nucl Med. 2011;25(5):339–345. doi: 10.1007/s12149-011-0471-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.