Abstract
Behcet's disease (BD) is a multisystem, autoimmune vasculitis disorder affecting small, medium, and large blood vessels, with poorly understood pathogenesis. It commonly presents with recurrent aphthous ulcers, genital ulcers, skin lesions, and bilateral uveitis. Neurological symptoms are present in less than 10% of cases and develop, on average, 5-6 years after the first non-neurological symptoms. This presentation, known as Neuro-Behcet's disease (NBD), is associated with a worse prognosis of BD. Treatment for NBD is dependent on the severity of symptoms and the presence of other systemic manifestations but often initially involves glucocorticoids and a disease-modifying agent. This case report presents a 44-year-old female patient, previously diagnosed with BD, who presented with neurological symptoms and MRI findings consistent with NBD.
Keywords: Neuro-Behcet disease, Behcet syndrome, Pons, Ventral medulla, Hyperintense punctate foci, MRI
Abbreviations: BD, Behcet's disease; NBD, Neuro-Behcet's disease; ED, emergency department; LE, lower extremity; UE, upper extremity; MRI, magnetic resonance imaging; LP, lumbar puncture; IV, intravenous; tPA, tissue plasminogen activator; NIH, National Institutes of Health; CT, computed tomography; CTA, computed tomography angiography; ANA, antinuclear antibodies; HLA, human leukocyte antigens; CSF, cerebrospinal fluid; CNS, central nervous system; MS, multiple sclerosis; RCVS, reversible cerebral vasoconstriction syndrome
Introduction
Behcet's disease (BD) is an autoimmune vasculitis, most commonly presenting with some combination of recurrent oral ulcers, genital ulcers, uveitis, and cutaneous lesions. Neurological symptoms are involved in less than 10% of BD cases, normally developing at least several years after initial diagnosis [1]. When present, they constitute a diagnosis of Neuro-Behcet's disease (NBD), which incorporates a broad range of possible symptoms, dependent on the specific location of neurological lesions. These lesions most often result from vasculitis-induced thrombosis [2] and are commonly found in the spinal cord, brainstem, basal ganglia, thalamus, or periventricular white matter. Diagnosis of NBD is aided by correlation of clinical symptoms, MRI findings, and CSF analysis. A timely diagnosis of NBD is vital, as treatment for BD is dependent on organ system involvement and severity of symptoms [3]. In this study, we present a patient with NBD with characteristic imaging and clinical findings.
Case report
A 44-year-old female with a history of migraines, stroke, and a diagnosis of BD 7 years prior, presented to the emergency department (ED) after awakening with increased right LE weakness. In addition, she reported increased urinary incontinence during the previous 3 days but no other new neurological symptoms. She was known to the neurology clinic for residual slurred speech, urinary incontinence, right-sided weakness, and bilateral hyperreflexia secondary to suspected stroke eleven months prior. An MRI of the brain was performed 3 months after this first stroke, demonstrating a chronic left pontine lacunar infarct and extensive white matter changes (Fig. 1). An MRI cervical spine and lumbar puncture (LP) was recommended for further work-up. However, neither study was performed due to insurance authorization issues, difficulty with IV access, and patient hesitancy.
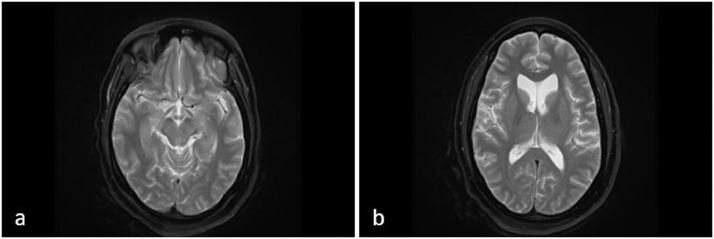
Fig. 1.
A 43-year-old female with right-sided weakness and a history of Bechet's disease. (A) T2-weighted MRI axial view at the level of the midbrain. (B) T2-weighted MRI axial view at the level of the lateral ventricle horns. Findings: Increased T2/FLAIR signal in the cerebral peduncles, ventral medulla, and middle cerebellar peduncle (A). Additional subtle scattered T2/FLAIR signal in the periventricular and deep cerebral white matter (B). Technique: Multiplanar, multisequence MRI-Brain/Stem was performed without administering contrast media.
A stroke evaluation was initiated during this ED visit and the patient was admitted for an inpatient neurology consult. Family history was notable for an aunt with multiple sclerosis and a mother with sarcoidosis. She reports the use of occasional alcohol and 1-2 joints of marijuana daily. Vital signs were normal. Physical exam was notable for mildly slurred speech and motor deficits, including mild right pronator drift, mild right UE weakness, mild weakness of right hip flexion, diffuse bilateral hyperreflexia, right-sided hemiparetic gait, and inability to perform heel-to-toe walking. Cranial nerves and sensation were intact. Lab results were unremarkable, and both a CT head and CTA head/neck were negative for acute intracranial process, early signs of a stroke, or large vessel occlusion.
An MRI of the brain was performed with and without intravenous contrast administration. MRI demonstrated markedly abnormal brainstem with expansile T2 hyperintensity involving almost the entire pons and extending into ventral medulla/medullary pyramids, midbrain, cerebral peduncles (more significant on the left), and posterior limb of the left internal capsule (Fig. 2 and Fig. 3) with significant interval progression compared to MRI performed 8 months prior. Additional MRI findings included a few nonspecific scattered punctate foci of T2 hyperintense signal in the cerebral white matter and a nonacute left corona radiata lacunar infarct, which was new from a prior study. Given the patient's history of BD, the findings are compatible with NBD.
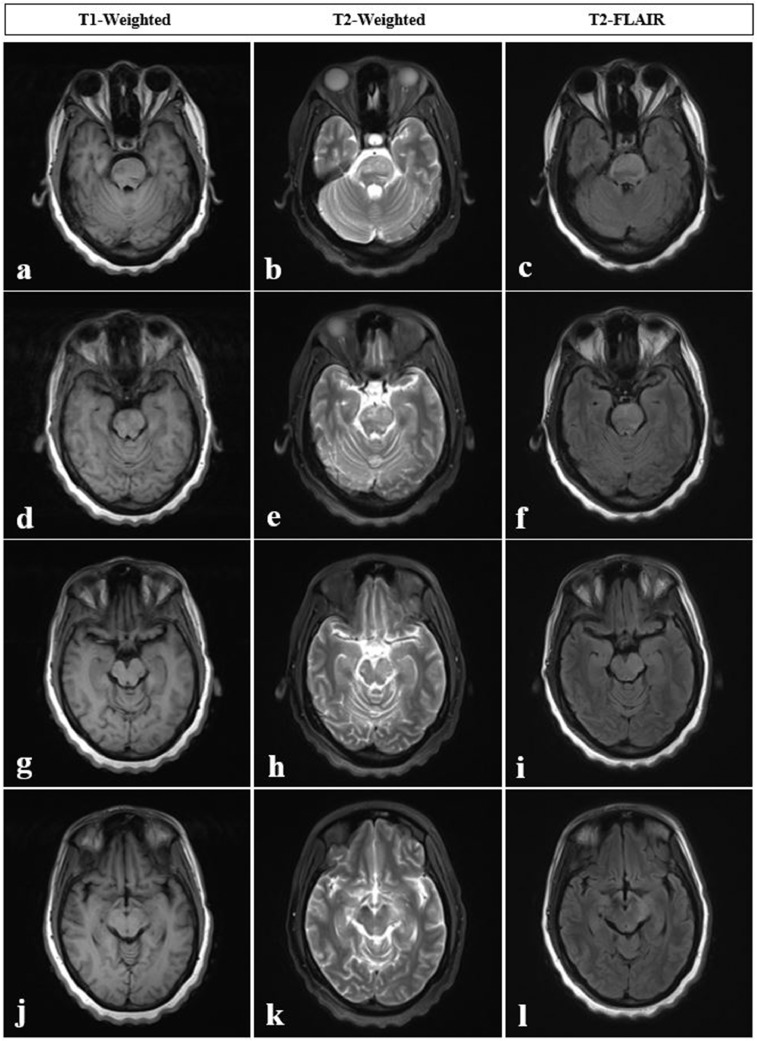
Fig. 2.
A 44-year-old female with worsening right-sided weakness and a history of Bechet’s disease, eight months after previous MRI images displayed in Figure 1. (A-F) MRI axial views of pons, using T1-weighted, T2-weighted, and FLAIR sequences. (G-L) MRI axial views of midbrain, using T1-weighted, T2-weighted, and FLAIR sequences. Findings: Markedly abnormal brainstem with expansile T2 hyperintense signal involving almost the entire pons (A-F), extending into ventral medulla/medullary pyramids, midbrain and cerebral peduncles (G-L) (asymmetrically greater on the left side), significantly progressed since the prior MRI. Technique: Multiplanar, multisequence MRI of the brain/brainstem was performed without and with intravenous contrast, 15 mL of Prohance.
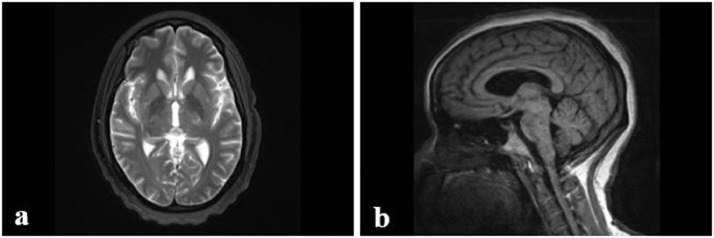
Fig. 3.
A 44-year-old female with worsening right-sided weakness and a history of Bechet’s disease. Additional imaging eight months after previous MRI images displayed in Figure 1. (A) T2-weighted MRI axial view of internal capsule. (B) T1-weighted MRI parasagittal view of the brain, left of midline. Findings: Markedly abnormal brainstem with expansile T2 hyperintense signal also extended to the posterior limb of the left internal capsule (A), significantly progressed since the prior MRI. Corresponding hypointense signal on T1-weighted parasagittal view, just left of midline (F). Technique: Multiplanar, multisequence MRI of the brain/brainstem was performed without and with intravenous contrast, 15 mL of Prohance.
The patient was discharged on IV corticosteroid therapy following a new diagnosis of NBD, awaiting insurance approval for monthly infusions of cyclophosphamide. Previous treatment for her BD included corticosteroids and methotrexate. She stopped taking her medications and discontinued follow-up with her rheumatologist 2 years prior to the ED visit, siting ineffective treatment of recurrent mouth ulcers. The patient's previous symptoms since her diagnosis of BD included oral sores, genital ulcers, and a single episode of uveitis.
At her 1-month postdischarge neurology clinic visit, she described considerable improvement in her mobility, right LE weakness, and urinary incontinence. However, she reported a recurrence of nonhealing oral ulcers. Physical exam was significant for subtle improvements in right LE strength with persistent hyperreflexia and mild right-sided weakness. New lab tests were significant for an ANA titer of 1:320, positive fluorescent ANA, and a positive pathergy test, all consistent with BD. The patient was instructed to continue current medications, including aspirin, atorvastatin, oral benzocaine jelly (for oral ulcers), IV steroid therapy, and cyclophosphamide.
Discussion
Neurological symptoms as a component of BD were first described in 1941 [4], and the term “Neuro-Behcet's” is credited to Italian ophthalmologists Cavara and D'Ermo in 1954 [5]. This disease can result in various CNS deficits, often associated with vascular thrombosis affecting the basal ganglia, brainstem, or spinal cord.
Etiology and pathogenesis
While the underlying cause of BD is unknown, its clinical manifestations are believed to result from autoimmune vasculitis, which uniquely may involve blood vessels of all sizes. Although it favors inflammation of venous vessels, it commonly manifests in the arterial system as well. Like other autoimmune diseases, BD results from an unrestrained immune response to an unknown trigger, thought to occur in people with a genetic predisposition for the disease [6]. NBD is believed to occur via the same pathogenesis as other systemic symptoms of BD. NBD can involve various areas of the CNS, including periventricular white matter, internal capsule, basal ganglia, thalamus, brainstem, cerebellum, and spinal cord.
Cross-reactivity between bacterial antigens and human peptides is suggested to play a role in the onset of BD. Specifically, mycobacterial heat shock proteins have a similar structure to human heat shock proteins, and elevated levels of antibodies against these bacterial epitopes have been seen in patients with BD [7]. For example, in one study, significant increases in gamma-delta T cells in response to mycobacterial heat shock proteins were present in 25 of 33 patients with BD, compared to just 2 of 55 in the control group [8].
The most well-known genetic marker associated with BD is HLA-B51/B5. One study showed carriers of this marker have a significantly increased risk of manifesting this disease, with a pooled odds ratio of 5.8 compared to non-carriers [9]. Other suspected etiologies of BD include aberrant neutrophil activation [10,11], vascular deposition of immune complexes [12,13], vascular endothelial activation [14,15], and epigenetic alterations [16].
Clinical and imaging findings
The classic presentation of BD includes the triad of recurring aphthous oral ulcers, genital ulcers, and uveitis. However, cutaneous lesions are also very common, occurring in over 75% of patients with the disease. Other less common symptoms associated with BD include pulmonary disease, venous thrombosis, arthritis, renal disease, cardiac disease, gastrointestinal involvement, fever, and neurological disease (NBD). Recurrent oral ulceration (>3 times in one year) is the most common presenting symptom of BD, and it often remits completely after about 20 years postinitial symptoms [17].
NBD presents in less than 10% of BD cases and is more frequent among males. Among those who develop NBD, symptoms first appear, on average, about 5-6 years after the onset of non-neurological symptoms [1]. There is a wide range of possible symptoms of NBD discussed in the literature, but they are most often due to focal parenchymal lesions associated with vascular thrombosis. Common clinical manifestations include motor dysfunction, memory impairment, and personality changes. Because the disease can potentially damage the spinal cord, brainstem, cerebellum, thalamus, basal ganglia, and internal capsule, symptoms are highly dependent on the location of the disease. Symptoms of NBD are often subacute in presentation, and deficits are compatible with lesions seen on MRI imaging, which often extend from the brainstem to the deep grey nuclei or result in noncontiguous signal alteration in the cervical spinal cord. These lesions can be unilateral or bilateral, and they are often 4-10 mm in diameter. Acute and subacute lesions present as hyperintense foci on T2-weighted images that enhance with contrast [18]. On MRI, chronic NBD lesions have little to no enhancement and demonstrate volume loss or brainstem atrophy. Lumbar puncture with NBD often reveals predominating neutrophils with increased protein and cells in the cerebrospinal fluid (CSF) [3].
In addition to parenchymal disease, NBD may also manifest with non-parenchymal disease, including thrombotic stroke, acute meningeal syndrome, and dural sinus thrombosis [2], all of which can be detected by MRI. In addition, thrombosis of the intracranial venous system may result clinically with increased CSF pressure, papilledema, and headache [19]. While parenchymal manifestations are more common among adults with NBD, evidence suggests that non-parenchymal symptoms are more common in children [20].
Treatment and prognosis
The general goal of treatment in BD is to promptly suppress inflammatory flares and limit recurrences to prevent permanent organ damage [21]. Because of the different systemic features present in any individual case, a multidisciplinary approach dependent on the manifested symptoms is recommended. Treatment can vary significantly based on age, gender, and the predominant organ system involved. Notably, BD with neurologic, gastrointestinal, or ocular components is associated with a worse prognosis. For milder BD cases presenting only with ulcers, corticosteroids and colchicine form the backbone of treatment, with immunomodulators being indicated for more severe disease.
NBD requires a different treatment approach due to the involvement of the CNS, but medication selection is still dependent on severity, other features of BD, and previous responsiveness to glucocorticoids [3]. The recommended first-line agent is azathioprine, and alternatives include cyclophosphamide, mycophenolate, and methotrexate. With encephalitis, medium-vessel vasculitis, or parenchymal lesions, high-dose corticosteroids and a TNF-alpha inhibitor should also be administered [22]. Some evidence suggests that cyclosporine has been associated with worsening of neurological symptoms in NBD [3], and it should thus be avoided in these patients. Additionally, anticoagulation may be considered in patients who have demonstrated ischemic vascular manifestations. There is no clear consensus on how long treatment should be administered after the suppression of symptoms in BD. It is generally recommended to taper corticosteroid therapy as tolerated and exchange potential toxic medications for those with better safety profiles. Some patients, especially those with severe cases such as NBD, will require life-long immunosuppressive therapy to control their disease adequately.
While BD limited to mucocutaneous symptoms has a generally favorable prognosis with possible eradication of symptoms and treatment discontinuation, NBD is associated with poorer outcomes. Neurological disease activity is thought to decline over time, but the cumulative burden of damage increases over the years due to the inability of neurons to regenerate. In addition, parenchymal disease is more likely to recur when compared to non-parenchymal disease. In one retrospective study of individuals with NBD, one-third of patients suffered a relapse, with the risk of relapse increased in those with the HLA-B51 marker. Clinical motor dysfunction and MRI-detected brainstem lesions were also associated with worse prognosis [23] (Table 1).
Table 1.
Summary table for neuro-Behcet's disease.
| Etiology | |
| Clinical Symptoms |
|
| MRI Findings |
|
| Treatment |
|
| Prognosis |
|
Differential diagnoses
Due to the variable presentation of NBD, the differential diagnosis may include a host of diseases affecting the CNS. Because NBD generally presents years after non-neurological findings of BD, the diagnosis is often easy to identify. The diagnosis may be more elusive, however, if neurological symptoms present as an isolated attack or prior to the identification of BD. Differential diagnoses of NBD include multiple sclerosis (MS), thromboembolic infarction, meningoencephalitis, and reversible cerebral vasoconstriction syndrome (RCVS).
-
•
Multiple sclerosis (MS): An autoimmune demyelinating disease of the CNS, characterized by multifocal loss of oligodendrocytes and astroglial scarring. Clinical symptoms often include optic neuritis, numbness, weakness, and paresthesia. MRI generally shows periventricular or juxtacortical ovoid lesions that vary by location over time [24]. T2-weighted MRI demonstrates lesions as hyperintense foci, failing to distinguish them from lesions present in NBD.
-
•
Thromboembolic infarction: Ischemic stroke due to etiology other than BD is often associated with an embolic source or risk factors for thrombosis. The most common embolic sources are atrial fibrillation and carotid stenosis, which could be evaluated by echocardiogram and CTA head/neck, respectively. Risk factors for thrombosis include smoking history, diabetes mellitus, hypertension, and hyperlipidemia. Diffusion-weighted imaging (DWI) demonstrates increased intracellular water in ischemic areas [25].
-
•
Reversible cerebral vasoconstriction syndrome (RCVS): A group of conditions which are all characterized by reversible constriction of cerebral arteries, with alternating areas of dilation. Clinical symptoms are normally acute in onset, including sudden headache, seizures, or focal neurologic deficits. Neuroimaging normally shows a “sausage on a string” appearance of arteries that compose the Circle of Willis and its branches. While MRI of the head is unremarkable in over 50% of cases, subarachnoid hemorrhage or symmetrical lesions in watershed areas may be identified [26] (Table 2).
Table 2.
Differential diagnosis table for neuro-Behcet's disease.
| Clinical findings | MRI | |
|---|---|---|
| Neuro-Behcet's disease | Motor dysfunction, dementia, personality changes | T2-weighted hyperintense foci, predilection for brainstem, basal ganglia, and thalamus [18] |
| Multiple sclerosis | Optic neuritis, numbness, weakness, paresthesia | T2-weighted hyperintense periventricular lesions that vary by location over time [24] |
| Thromboembolic infarction | Acute, unilateral focal deficits | Increased intracellular water demonstrated by DWI in ischemic area [25] |
| RCVS | Headache, seizures, bilateral focal deficits | “Sausage on a string” appearance of arteries in Circle of Willis and its branches [26] |
Conclusion
In patients with nonspecific neurological deficits and medium sized lesions in the brainstem or basal ganglia identified on MRI, the potential diagnosis of NBD should be considered. Suspected NBD in a patient without a previous diagnosis of BD should elicit a work-up for systemic manifestations, including a guided history and physical exam of the mouth, genitals, eyes, and skin.
Patient consent
Written, informed consent for publication of this case study was obtained from the patient.
Footnotes
Competing Interests: All authors declare no conflict of interest.
References
- 1.Akman-Demir G, Serdaroglu P, Tasçi B. Clinical patterns of neurological involvement in Behçet's disease: evaluation of 200 patients. The Neuro-Behçet Study Group. Brain. 1999;122(Pt 11):2171. doi: 10.1093/brain/122.11.2171. [DOI] [PubMed] [Google Scholar]
- 2.Farah S, Al-Shubaili A, Montaser A, Hussein JM, Malaviya AN, Mukhtar M, et al. Behçet's syndrome: a report of 41 patients with emphasis on neurological manifestations. J Neurology Neurosurgery Psychiatry. 1998;64:382. doi: 10.1136/jnnp.64.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalra S, Silman A, Akman-Demir G, Bohlega S, Borhani-Haghighi A, Constantinescu CS, et al. Diagnosis and management of Neuro-Behçet's disease: international consensus recommendations. J Neurology. 2014;261:1662. doi: 10.1007/s00415-013-7209-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knapp P. Beitrag zur Symptomatologie und Therapie der rezidivierenden Hypopioniritis und der begleitenden aphtösen Schleimhauterkrankungen. Schweiz Med Wochenschr. 1941;71:1288–1290. [Google Scholar]
- 5.Al-Araji A, Kidd DP. Neuro-Behçet's disease. Epidemiology, clinical characteristics and management. Lancet Neurology. 2009;8:192–204. doi: 10.1016/S1474-4422(09)70015-8. [DOI] [PubMed] [Google Scholar]
- 6.Direskeneli H. Behçet's disease. infectious aetiology, new autoantigens, and HLA-B51. Ann Rheum Disease. 2001;60:996. doi: 10.1136/ard.60.11.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Direskeneli H, Hasan A, Shinnick T, Mizushima R, van der Zee R, Fortune F, et al. Recognition of B-cell epitopes of the 65 kDa HSP in Behçet's disease. Scand J Immunology. 1996;43:464. doi: 10.1046/j.1365-3083.1996.d01-53.x. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Hoshi K, Matsuda T, Mizushima Y. Increased peripheral blood gamma delta+ T cells and natural killer cells in Behçet's disease. J Rheumatology. 1992;19:588. [PubMed] [Google Scholar]
- 9.de Menthon M, Lavalley MP, Maldini C, Guillevin L, Mahr A. HLA-B51/B5 and the risk of Behçet's disease: a systematic review and meta-analysis of case-control genetic association studies. Arthritis Rheum. 2009;61:1287. doi: 10.1002/art.24642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raziuddin S, Dalaan A, Bahabri S, Siraj AK, Sedairy S. Divergent cytokine production profile in Behçet's disease. Altered Th1/Th2 cell cytokine pattern. J Rheumatology. 1998;25:329. [PubMed] [Google Scholar]
- 11.Triolo G, Accardo-Palumbo A, Triolo G, Carbone MC, Ferrante A, Giardina E. Enhancement of endothelial cell E-selectin expression by sera from patients with active Behçet's disease: moderate correlation with anti-endothelial cell antibodies and serum myeloperoxidase levels. Clinical Immunology. 1999;91:330. doi: 10.1006/clim.1999.4687. [DOI] [PubMed] [Google Scholar]
- 12.Evereklioglu C. Current concepts in the etiology and treatment of Behçet disease. Survey Ophthalmology. 2005;50:297. doi: 10.1016/j.survophthal.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Ekşioglu-Demiralp E, Kibaroglu A, Direskeneli H, Yavuz S, Karsli F, Yurdakul S, et al. Phenotypic characteristics of B cells in Behçet's disease: increased activity in B cell subsets. J Rheumatology. 1999;26:826. [PubMed] [Google Scholar]
- 14.Oflaz H, Mercanoglu F, Karaman O, Kamali S, Erer B, Genchellac H, et al. Impaired endothelium-dependent flow-mediated dilation in Behçet's disease: more prominent endothelial dysfunction in patients with vascular involvement. Int J Clinical Practice. 2005;59:777. doi: 10.1111/j.1742-1241.2005.00477.x. [DOI] [PubMed] [Google Scholar]
- 15.Kayikçioğlu M, Aksu K, Hasdemir C, Keser G, Turgan N, Kültürsay H, et al. Endothelial functions in Behçet's disease. Rheumatology Int. 2006;26:304. doi: 10.1007/s00296-005-0590-1. [DOI] [PubMed] [Google Scholar]
- 16.Alipour S, Nouri M, Sakhinia E, Samadi N, Roshanravan N, Ghavami A, et al. Epigenetic alterations in chronic disease focusing on Behçet's disease. Review. Biomed Pharmacotherapy. 2017;91:526. doi: 10.1016/j.biopha.2017.04.106. [DOI] [PubMed] [Google Scholar]
- 17.O'Duffy JD. (1993). Behcet's syndrome. In: Primer on the Rheumatic Diseases, 10th, Arthritis Foundation, Atlanta, Vol 29, p.206.
- 18.Lee SH, Yoon PH, Park SJ, Kim DI. MRI findings in neuro-behçet's disease. Clinical Radiology. 2001;56:485. doi: 10.1053/crad.2000.0675. [DOI] [PubMed] [Google Scholar]
- 19.Shi J, Huang X, Li G, Wang L, Liu J, Xu Y, et al. Cerebral venous sinus thrombosis in Behçet's disease: a retrospective case-control study. Clinical Rheumatology. 2018;37:51. doi: 10.1007/s10067-017-3718-2. [DOI] [PubMed] [Google Scholar]
- 20.Uluduz D, Kürtüncü M, Yapıcı Z, Seyahi E, Kasapçopur Ö, Özdogan H, et al. Clinical characteristics of pediatric-onset neuro-Behçet disease. Neurology. 2011;77:1900. doi: 10.1212/WNL.0b013e318238edeb. [DOI] [PubMed] [Google Scholar]
- 21.Hatemi G, Christensen R, Bang D, Bodaghi B, Celik AF, Fortune F, et al. 2018 update of the EULAR recommendations for the management of Behçet's syndrome. Ann Rheum Disease. 2018;77:808. doi: 10.1136/annrheumdis-2018-213225. [DOI] [PubMed] [Google Scholar]
- 22.Yalcin Kehribar D, Gunaydin S, Ozgen M. Infliximab therapy in parenchymal neuro-Behçet's disease: A single-center experience. Int J Rheum Disease. 2021;24:1302. doi: 10.1111/1756-185X.14209. [DOI] [PubMed] [Google Scholar]
- 23.Noel N, Bernard R, Wechsler B, Resche-Rigon M, Depaz R, Boutin D, et al. Long-term outcome of neuro-Behçet's disease. Arthritis Rheumatology. 2014;66:1306. doi: 10.1002/art.38351. [DOI] [PubMed] [Google Scholar]
- 24.Diri E, Espinoza LR. Neuro-Behçet's syndrome: differential diagnosis and management. Curr Rheumatology Rep. 2006;8(4):317–322. doi: 10.1007/s11926-006-0016-4. [DOI] [PubMed] [Google Scholar]
- 25.Beauchamp NJ, Jr, Barker PB, Wang PY, vanZijl PC. Imaging of acute cerebral ischemia. Radiology. 1999;212:307. doi: 10.1148/radiology.212.2.r99au16307. [DOI] [PubMed] [Google Scholar]
- 26.Ducros A, Boukobza M, Porcher R, Sarov M, Valade D, Bousser M. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091. doi: 10.1093/brain/awm256. [DOI] [PubMed] [Google Scholar]