Abstract
The limited performance of guideline‐recommended abdominal ultrasound and serum alpha‐fetoprotein (AFP) highlights the urgent, unmet need for new biomarkers for more accurate detection of early hepatocellular carcinoma (HCC). To this end, we have conducted a prospective clinical validation study to evaluate the performance of the HelioLiver Test, a multi‐analyte blood test combining cell‐free DNA methylation patterns, clinical variables, and protein tumor markers. A blinded, multicenter validation study was performed with 247 subjects, including 122 subjects with HCC and 125 control subjects with chronic liver disease. The performance of the HelioLiver Test was compared with AFP and the GALAD score as established HCC surveillance blood tests. The performance of the HelioLiver Test (area under the receiver operating characteristic curve [AUROC] = 0.944) was superior to both AFP (AUROC = 0.851; p < 0.0001) and GALAD (AUROC = 0.899; p < 0.0001). Using a prespecified diagnostic algorithm, the HelioLiver Test showed sensitivities of 85% (95% confidence interval [CI], 78%–90%) for HCC of any stage and 76% (95% CI, 60%–87%) for early stage (American Joint Committee on Cancer [AJCC] I and II) HCC. In contrast, AFP (≥20 ng/mL) alone and the GALAD score (≥−0.63) showed lower sensitivities of 62% (95% CI, 54%–70%) and 75% (95% CI, 67%‐82%) for HCC overall, and 57% (95% CI, 40%–71%) and 65% (95% CI, 49%–79%) for early stage (AJCC I and II) HCC, respectively. The specificities of the HelioLiver Test (91%; 95% CI, 85%–95%), AFP (97%; 95% CI, 92%–99%), and the GALAD score (94%; 95% CI, 88%–97%) were similar for control subjects. The HelioLiver Test showed superior performance for HCC detection compared to with both AFP and the GALAD score and warrants further evaluation in HCC surveillance settings.
The limited performance of guideline‐recommended abdominal ultrasound and serum alpha‐fetoprotein (AFP) highlights the urgent, unmet need for new biomarkers for more accurate detection of early hepatocellular carcinoma (HCC). To this end, we have conducted a prospective clinical validation study to evaluate the performance of the HelioLiver Test, a multi‐analyte blood test combining cell‐free DNA (cfDNA) methylation patterns, clinical variables, and protein tumor markers. The HelioLiver Test showed superior peformacne for HCC detection compared to both AFP and the GALAD score and warrants further evaluation in HCC surveillance settings.

INTRODUCTION
Mortality of liver cancer, with hepatocellular carcinoma (HCC) as the major histological type, has substantially increased over the past two to three decades in the United States and worldwide, and its incidence is projected to continue to increase over the next decade.[ 1 , 2 , 3 ] HCC prognosis remains dismal (5‐year survival <15%) due to frequent diagnoses at late, noncurable stages.[ 4 , 5 ]
To increase HCC tumor detection at earlier stages that are more amenable to curative treatment, practice guidelines recommend semi‐annual HCC surveillance using ultrasound with or without serum alpha‐fetoprotein (AFP) for high‐risk populations.[ 6 , 7 , 8 ] However, the suboptimal performance of current HCC surveillance tests hinders effective early tumor detection. The sensitivity of ultrasound for detecting early‐stage HCC is only 45%, and a systematic review and meta‐analysis study has suggested that the improvement to ultrasound by adding AFP is limited to only 63%.[ 9 ] Additionally, the performance of ultrasound is reduced by various factors such as obesity, which is sharply increasing globally.[ 10 , 11 ] Thus, HCC surveillance tests with superior performance are urgently needed. In an effort to improve HCC detection, the GALAD score was developed by combining age, sex, AFP, Lens culinaris agglutinin‐reactive AFP (AFP‐L3%), and des‐gamma‐carboxy prothrombin (DCP).[ 12 ] The GALAD score has shown superior HCC detection performance compared with AFP, but there is room for improvement,[ 13 , 14 ] indicating the still unmet need for HCC surveillance tests with substantially improved performance characteristics.
“Liquid biopsies” assaying the methylation of circulating cell‐free DNA (cfDNA) released from cancer cells have been actively explored as a promising noninvasive biomarker to sensitively detect various cancer types, including HCC, at early stages.[ 15 ] Previously, from a comprehensive methylome profiling of HCC tissue/plasma samples combined with machine‐learning analysis, we identified cfDNA methylation markers associated with the presence of HCC in patients with chronic liver diseases as a potential HCC detection biomarker.[ 16 , 17 ] Based on this foundational work, we further optimized the candidate cfDNA methylation panel and developed the HelioLiver Test, a multi‐analyte blood test that combines cfDNA methylation markers with patient demographic information and clinically available HCC tumor markers (components of the GALAD score) to enable robust and accurate HCC detection. Here, we validated the performance characteristics of the HelioLiver Test in a prospective, blinded, multi‐center phase 2 biomarker study (the ENCORE study; NCT05059665).
PATIENTS AND METHODS
Study design
The HelioLiver Test was prospectively validated in an independent, multicenter study (the HelioLiver Test Validation Set 1) for the blinded evaluation of test performance as an HCC early detection marker (The Specimen Collection for Multi‐analyte Blood Test for Hepatocellular Carcinoma [ENCORE] study; NCT05059665). The full study details can be accessed on clinicaltrials.gov.
Study subjects
Subjects recruited in this study were patients newly diagnosed with HCC or patients with a benign liver disease that were recommended for HCC surveillance and were found to be without HCC (control subjects). Subjects with HCC were diagnosed by histopathologic examination or by specific radiologic characteristics according to current practice guidelines in China.[ 18 ] HCC stage (i.e., extent of tumor spread) was determined for subjects according to the American Joint Commission on Cancer (AJCC) 8th Edition. The control subjects were patients who were recommended to HCC surveillance in China[ 18 ] due to underlying chronic liver disease, including chronic fibrotic liver diseases from any cause, chronic hepatitis B virus (HBV) infection, chronic hepatitis C virus infection, fatty liver disease, and nonalcoholic fatty liver disease.
The presence of cirrhosis was defined by histology or clinical evidence of portal hypertension in subjects with chronic liver disease. All clinical information, including patient demographics and clinical characteristics, were prospectively obtained from medical records.
All subjects were prospectively and consecutively enrolled at the Third Affiliated Hospital of Sun Yat‐sen University (Guangzhou, China) and the First Affiliate Hospital of Guangzhou Medical University (Guangzhou, China) between 2020 and 2021 with written informed consent. The study was approved by their respective ethical review boards.
In total, the study included 140 patients with HCC and 150 patients diagnosed with a benign liver disease without HCC (control subjects). A total of 93 subjects were enrolled at the Third Affiliated Hospital of Sun Yat‐sen University, and 210 subjects were enrolled at the First Affiliate Hospital of Guangzhou Medical University. Subsequently, 5 subjects were excluded for incomplete health and/or demographic information, and 44 subjects were excluded for failing to meet quality control criteria for the HelioLiver Test, specifying an average sequencing coverage of ≥50 times among all target sites. The final study population analyzed consisted of 122 patients with HCC and 125 control subjects (Table 1).
TABLE 1.
Clinical characteristics of study participants
| HCC | Control subjects | |
|---|---|---|
| Subjects (n) | 122 | 125 |
| Age, median, years | 55 | 47 |
| Sex | ||
| Male (n) | 106 (87%) | 83 (66%) |
| Female (n) | 16 (13%) | 42 (34%) |
| Liver disease | ||
| Cirrhosis, n (%) | 45 (37%) | 46 (37%) |
| Chronic HBV, n (%) | 88 (72%) | 72 (58%) |
| Chronic HCV, n (%) | 3 (3%) | 4 (3%) |
| Fatty liver disease, n (%) | 1 (1%) | 20 (16%) |
| Other a , n (%) | 34 (28%) | 26 (21%) |
| Protein tumor markers | ||
| AFP, median (ng/mL) | 65.8 | 1.7 |
| AFP‐L3%, median (%) | 10% | <5% |
| DCP, median (ng/mL) | 13.8 | 0.6 |
| GALAD | 2.76 | −4.52 |
| Stage | ||
| I | 29 (24%) | |
| II | 8 (7%) | |
| III | 43 (35%) | |
| IV | 28 (23%) | |
| Unstaged or unknown | 14 (12%) |
Abbreviations: HBV, hepatitis B virus; HCV, hepatitis C virus.
Other liver conditions include liver cysts and benign liver tumors.
Serum protein HCC tumor markers
Serum concentrations of AFP, AFP‐L3%, and DCP were measured by using commercially available assays (Hotgen Biotech, Beijing, China) on a HotGen MQ60 instrument according to the manufacturer’s instructions.
cfDNA methylation detection with targeted capture assay
The Helios Eclipse platform was used to evaluate methylation patterns of cfDNA at target sites (Table S1). To this end, total cfDNA was isolated from specimens by using the EliteHealth cfDNA Extraction Kit (EliteHealth, Guangzhou Youze, China). Isolated cfDNA was eluted into nuclease‐free low‐bind 1.5‐mL microcentrifuge tubes and stored at −80°C. DNA concentration was measured using the Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific, USA) as per manufacturer’s instructions. A total of 5 ng cfDNA per sample was used to prepare the barcoded next‐generation sequencing (NGS) libraries by using the NEB Next Enzymatic Methyl‐seq Kit (New England Biolabs, USA) according to the manufacturer’s instructions. The libraries were then pooled in groups of 24 barcoded libraries at 100 ng each and hybridized with a custom set of HelioLiver capture probes (Twist Bioscience, USA) to capture the target library sequences using the Twist Fast Hybridization and Wash Kit, along with the Twist Universal Blocker. The captured libraries were then supplemented with 20% PhiX genomic DNA library to increase base calling diversity and submitted for NGS on either a HiSeq X or a NovaSeq 6000 platform (Illumina, USA).
cfDNA methylation data analysis
Raw sequencing data were first trimmed by TrimGalore (ver. 0.6.5) to remove low‐quality (Phred score < 20) sequences and potential adapter contamination. To remove M‐bias, 5 bp and 10 bp of sequence was trimmed from the 5’ end of Read 1 and Read 2, respectively. Cleaned sequencing reads were then aligned to the hg19 human reference genome by using BSMAP (ver. 2.90).[ 19 ] The aligned reads were further processed by Samtools (ver. 1.13)[ 20 ] and Bedtools (ver. 2.29.1)[ 21 ] to select only primarily mapped reads with fragment size between 80 bp and 200 bp. Methratio.py (BSMAP) was finally used to extract the methylation ratio from aligned bam files. Samples with insufficient sequencing depth (<50 times) were excluded from the downstream analysis.
HelioLiver Test
The HelioLiver Test (Figure 1) was developed to discriminate between patients with HCC from high‐risk patients without HCC. Figure 2 summarizes the overall HelioLiver Test development. Briefly, DNA methylation sites that were shown to be differentially methylated between HCC and control subjects for tissue and blood specimens were first identified from The Cancer Genome Atlas and evaluated in previous studies.[ 16 , 17 ] These target DNA methylation sites were included in a Discovery methylated cell‐free DNA (m‐cfDNA) panel. DNA methylation sites with undesirable characteristics (e.g., repeated elements, poor sequence capture) in the NGS‐based cfDNA methylation assay were then removed to generate a preliminary NGS m‐cfDNA panel.
FIGURE 1.
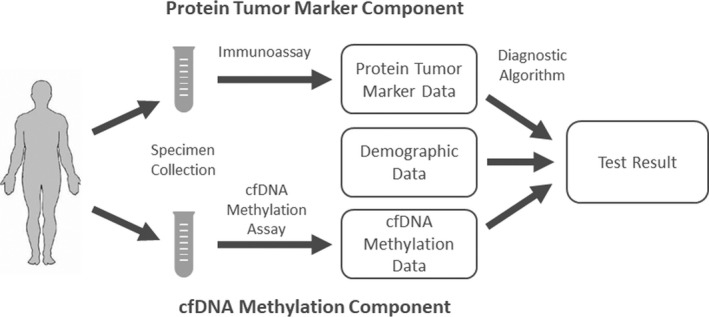
The HelioLiver Test Workflow. Blood specimens were collected from patients and then assayed for alpha‐fetoprotein (AFP), Lens culinaris agglutinin‐reactive AFP (AFP‐L3%), and des‐gamma‐carboxy prothrombin (DCP) by using immunoassays, and for cell‐free DNA (cfDNA) methylation patterns of 28 genomic regions by using a cfDNA Methylation Assay. The data for each assay were then input into a diagnostic algorithm with the patient’s age and sex, to generate a qualitative (positive/negative) test result
FIGURE 2.
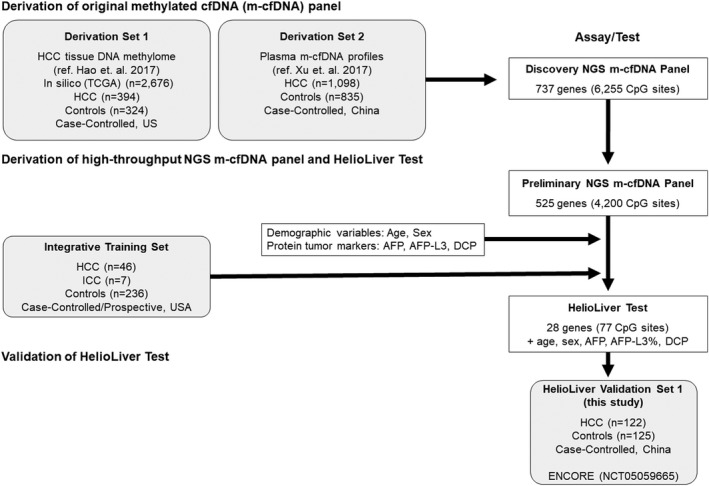
Development of the HelioLiver Test. To develop the HelioLiver Test, DNA methylation markers that were consistently differentially methylated by hepatocellular carcinoma (HCC) were first identified in both tissue and blood specimens (Derivation Set 1 and Derivation Set 2), yielding the Discovery cfDNA methylation (m‐cfDNA) panel. This Discovery m‐cfDNA panel was then refined to exclude markers with undesirable characteristics (repeated elements, poor capture) to generate the Preliminary m‐cfDNA panel. The Preliminary next‐generation sequencing (NGS) m‐cfDNA panel was then combined with protein tumor markers (AFP, AFP‐L3%, and DCP) and patient demographic data (age and sex) within an Integrative Training Data Set, to train the HelioLiver Test diagnostic algorithm. The final HelioLiver Test consists of 28 gene (77 CpG site) m‐cfDNA markers, three protein tumor markers (AFP, AFP‐L3%, and DCP), and patient demographic characteristics (age and sex). The HelioLiver Test was then evaluated within HelioLiver Validation Set 1 (described in this manuscript; ENCORE study; NCT05059665). White boxes indicate test development steps. Gray boxes represent sample sets. ICC, intrahepatic cholangiocarcinoma
Finally, an optimized subset of m‐cfDNA markers, clinically available serum protein markers (AFP, AFP‐L3%, and DCP), and patient demographics (age and sex) were combined to generate the HelioLiver Test. To this end, we first selected cytosine‐guanine dinucleotide (CpG) sites showing significant methylation alteration in HCC samples compared to non‐HCC control samples. Subsequently, the feature selection R package “Boruta” was used to identify the optimal cfDNA methylation markers within the Integrative Training Set. This approach identified 77 CpG sites in 28 genes (Table S1) as being significantly and consistently differentially methylated for HCC and was used to construct the cfDNA methylation model. For model training, we assessed different off‐the‐shelf machine learning models and chose the random forest model (implemented by R package “Ranger”) that showed the best performance. The hyper parameters of the random forest model were fine‐tuned by the grid‐search method. The cfDNA methylation component, protein tumor marker component, and demographic component were combined by using a decision tree model to generate the HelioLiver Test diagnostic algorithm. The threshold of the HelioLiver Test diagnostic algorithm was fixed based on the out‐of‐bag predictions in the Training Set to achieve approximately 90% specificity. The HelioLiver diagnostic algorithm was then locked before the initiation of this validation study (ENCORE). For cfDNA methylation analysis, targeted NGS capture was performed by using the Preliminary NGS m‐cfDNA panel. However, only the 28 target genes (77 CpG sites) included in the HelioLiver Test (Table S1) were used to calculate HelioLiver Test results.
Statistical analysis
For the independent clinical validation of the HelioLiver Test (in Validation Set 1), the primary endpoint was to compare the area under receiver operating characteristic (AUROC) curve of the HelioLiver Test to both AFP alone and the GALAD score. The co‐secondary endpoints were to compare the sensitivity and specificity of the HelioLiver Test (using a prespecified diagnostic algorithm and cutoffs) to AFP at the most commonly reported clinical cutoff of 20 ng/mL,[ 22 ] at a lower cutoff of 10 ng/mL, and to the GALAD score at a proposed cutoff of −0.63.[ 12 ] As an exploratory endpoint, the sensitivity of the HelioLiver Test was compared with AFP and the GALAD score at standardized specificities. As a post hoc analysis, the performance characteristics of AFP‐L3% alone, DCP alone, and the combination of AFP and DCP[ 23 ] were also calculated for comparison. Due to the relatively high prevalence of chronic HBV within the study population, a post hoc subgroup analysis was additionally performed in a subpopulation of subjects without chronic HBV infection, to compare the AUROC curve, sensitivity, and specificity of the HelioLiver Test, AFP alone, and the GALAD score.
The comparison of the AUROCs for both all subjects with HCC and only early (stage I and II) HCC were performed by sample permutation–based Wilcoxon signed‐rank test (10,000 permutations) with Bonferroni correction. The comparisons of the sensitivity and specificity of the HelioLiver Test to AFP and GALAD score were performed using McNemar’s test for paired proportions. A two‐tailed p value less than 0.05 was regarded as statistically significant. All statistical analyses were performed by using Prism software version 8.0 (GraphPad, La Jolla, CA). To assess confounding, the logit function from the python statsmodels module (statsmodels.formula.api.logit) was used to perform logistic regression, with the cancer status as the response variable, and the HelioLiver Test result along with age, gender, and several benign liver conditions as explanatory variables. For each variable, the exponential of the coefficient was calculated to determine the odds ratio.
RESULTS
Development of the HelioLiver Test and algorithm
The HelioLiver Test algorithm was developed as outlined in Figure 2. Interestingly, 10 of the 28 genes in our cfDNA panel are involved in molecular pathways implicated in HCC pathogenesis,[ 24 , 25 ] whereas of the 497 unselected genes, only MLK1[ 26 ] has been associated in molecular pathways implicated in HCC pathogenesis (Figure S1).
Independent validation of the HelioLiver Test (the ENCORE study)
We prospectively enrolled 247 evaluable subjects (Figure 3), including 122 subjects diagnosed with HCC and 125 subjects with a chronic liver disease, who were found to be without HCC after undergoing HCC surveillance (control subjects). The demographic and clinical characteristics of all eligible subjects are described in Table 1. The subjects with HCC were older (median age = 55 years) compared with the control subjects (median age = 47 years). The major disease etiology was HBV infection similarly among both subjects with HCC (72%) and control subjects (58%), in part due to the high rate of HBV infections in China. As expected, AFP, AFP‐L3%, DCP, and the GALAD score were higher in the subjects with HCC compared to the control subjects. The individual performance of the 525 genes included in the Preliminary NGS cfDNA panel used to evaluate ENCORE subjects and the performance of the 28 genes included in the HelioLiver Test are described in Table S1.
FIGURE 3.
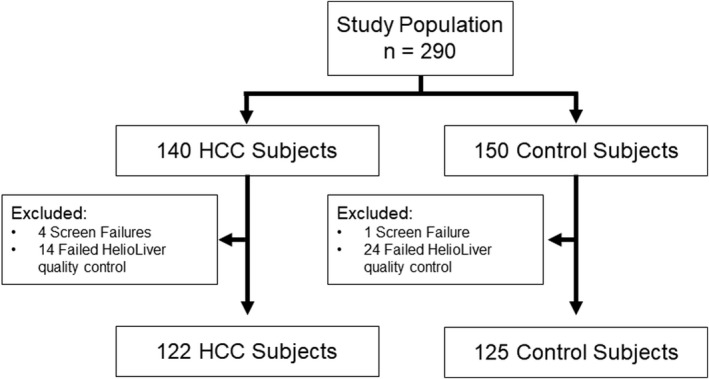
ENCORE validation study workflow
Performance of the HelioLiver Test for HCC detection compared with AFP and GALAD
As the primary endpoint of the study, AUROC curves were used to compare the performance characteristics of the HelioLiver Test to both AFP alone and the GALAD score for the detection of HCC (Figure 4). The HelioLiver Test demonstrated a significantly higher AUROC of 0.944 (95% CI 0.917–0.975) compared with AFP (AUROC 0.851; 95% CI 0.777–0.903; p < 0.0001), AFP‐L3% (AUROC 0.801; 95% CI 0.755–0.847; p < 0.0001), DCP (AUROC 0.780; 95% CI 0.719–0.842; p < 0.0001), and the GALAD score (AUROC 0.899; 95% CI 0.833–0.941; p < 0.0001) for the detection of HCC overall (Figure 4A). The HelioLiver Test (AUROC 0.924; 95% CI 0.846–0.986) also outperformed both AFP (AUROC 0.806; 95% CI 0.653–0902; p < 0.0001), AFP‐L3% (AUROC 0.769; 95% CI 0.686–0.852; p < 0.0001), DCP (AUROC 0.742; 95% CI 0.632–0.852; p < 0.0001), and the GALAD score (AUROC 0.842; 95% CI 0.693–0.926; p = 0.0003) for the detection of early‐stage (AJCC stage I and II) HCC (Figure 4B). As anticipated, the performance of GALAD was superior to AFP, AFP‐L3%, and DCP alone for detection of both HCC overall and early HCC (Figure 4).
FIGURE 4.
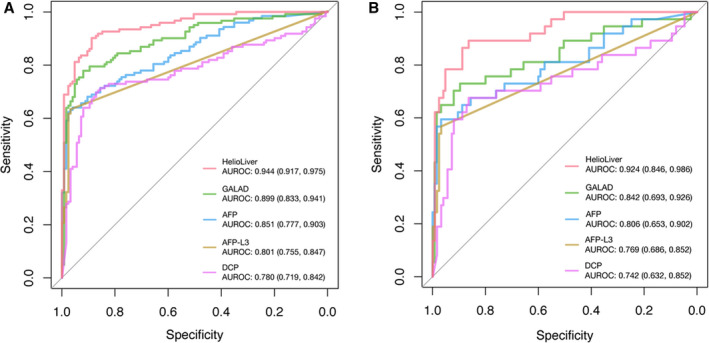
Receiver operating characteristic (ROC) curves for HCC blood tests. (A) ROC curves for analysis of all subjects diagnosed with HCC and control (benign liver disease) subjects. (B) Subjects diagnosed with early‐stage (American Joint Commission on Cancer [AJCC] stage I and II) HCC and control (benign liver disease) subjects. AUROC, area under receiver operating characteristic
To investigate whether confounding variables influenced the HelioLiver Test results, we used logistic regression analysis to assess the relationship between the patient group (subjects with HCC or control subjects) and the HelioLiver Test result in the presence of potential confounding variables including age, gender, and underlying liver disease. We then calculated an odds ratio for the HelioLiver Test result, adjusted for these potential confounders (Table S2). The coefficient associated with the HelioLiver Test prediction was calculated to be 3.9 with a p value < 2.2e‐16 and an odds ratio = 50. This suggests that patients that have a positive HelioLiver Test are approximately 50 times more likely to actually have HCC than patients with a negative test result. This odds ratio was adjusted for patient demographic data (age and gender) and the underlying liver disease of the subjects.
To further confirm that the underlying etiology of liver disease for ENCORE subjects did not influence the performance characteristics of the HelioLiver Test, a subset of 100 subjects diagnosed with HCC and 100 control subjects with matched liver disease etiologies was identified (Table S3). Within the etiology‐matched subgroup of subjects, the HelioLiver Test demonstrated superior performance characteristics (Table S4 ) for HCC overall (AUROC 0.933; 95% CI 0.905–0.964) compared with AFP (AUROC 0.844; 95% CI 0.789–0.898), AFP‐L3% (AUROC 0.797; 95% CI 0.745–0.848; p < 0.0001), DCP (AUROC 0.750; 95% CI 0.678–0.821; p < 0.0001), and the GALAD score (AUROC 0.881; 95% CI 0.832–0.930) (Figure S2). The HelioLiver Test (AUROC 0.917; 95% CI 0.866–0.968) similarly outperformed the AFP (AUROC 0.803; 95% CI 0.708–0.898), AFP‐L3% (AUROC 0.765; 95% CI 0.682–0.849; p < 0.0001), DCP (AUROC 0.733; 95% CI 0.622–0.844; p < 0.0001), and GALAD score (AUROC 0.834; 95% CI 0.743–0.924) for the detection of early (Stage I and II) HCC within the etiology matched subgroup of subjects.
As co‐secondary endpoints, the sensitivity and specificity of the HelioLiver Test (using a prespecified diagnostic algorithm and cutoffs) were compared with GALAD and the individual protein tumor markers at standard clinical cutoffs. These test performance characteristics are summarized in Table 2. The HelioLiver Test (85.2%; 95% CI 77.8%–90.4%) demonstrated a superior overall sensitivity for the detection of all‐stage HCC compared with AFP at both the commonly used cutoff 20 ng/mL (62.3%; 95% CI 53.5%–70.4%) and a lower cutoff of 10 ng/mL (68.0%; 95% CI 59.3%–75.6%). The HelioLiver Test was also more sensitive than the GALAD score at an established cutoff of −0.63 (75.4%: 95% CI 67.1%–82.2%). The HelioLiver Test demonstrated a superior sensitivity (75.7%; 95% CI 59.9%–86.7%) for early‐stage (I and II) HCC when compared with AFP at both the 20‐ng/mL cutoff (56.8%; 95% CI 40.1%–71.4%) and the 10‐ng/mL cutoff (62.2%; 95% CI 46.1%–76.0%), and the GALAD score (64.9%; 95% CI 48.8%–78.2%) at the cutoff of −0.63. The specificity of the HelioLiver Test (91.2%; 95% CI 84.9%–95.0%) was comparable to AFP at the 10‐ng/mL cutoff (90.4%; 95% CI 84.0–94.4%) and the GALAD score (93.6%; 95% CI 87.9%–96.7%). The sensitivity of both the HelioLiver Test and the GALAD score (at both the −0.63 and −1.2 cutoffs) was found to be superior to AFP‐L3% (≥10% cutoff), DCP (≥7.5 ng/mL cutoff), and the combination of AFP (≥20 ng/mL cutoff) and DCP (≥7.5 ng/mL cutoff)[ 23 ] for the detection of both HCC overall and early‐stage HCC (Table 2).
TABLE 2.
Comparison of test performance characteristics for detection of HCC
| Early‐stage (I + II) sensitivity, % (95% CI) (n = 37) | Late‐stage (III + IV) sensitivity, % (95% CI) (n = 71) | Overall sensitivity, % (95% CI) (n = 122) | Specificity, % (95% CI) (n = 125) | |
|---|---|---|---|---|
| HelioLiver Test | 75.7 (59.9, 86.7) | 91.5 (85.2, 95.3) | 85.2 (77.8, 90.4) | 91.2 (84.9, 95.0) |
| GALAD (≥−0.63) | 64.9 (48.8, 78.2) | 80.3 (72.4, 86.4) | 75.4 (67.1, 82.2) | 93.6 (87.9, 96.7) |
| GALAD (≥−1.2) a | 70.3 (54.3, 82.5) | 81.7 (73.9, 87.6) | 77.9 (69.8, 84.4) | 91.2 (84.9, 95.0) |
| AFP (≥10 ng/mL) | 62.2 (46.1, 76.0) | 69.0 (57.5, 78.6) | 68.0 (59.3, 75.6) | 90.4 (84.0, 94.4) |
| AFP (≥12.1 ng/mL) a | 59.5 (43.5, 73.4) | 67.6 (58.9, 75.3) | 66.4 (57.6, 74.2) | 91.2 (84.9, 95.0) |
| AFP (≥20 ng/mL) | 56.8 (40.1, 71.4) | 63.4 (54.6, 71.4) | 62.3 (53.5, 70.4) | 96.8 (92.1, 98.8) |
| AFP‐L3% (≥10%) | 51.4 (35.9, 66.6) | 60.6 (49.0, 71.1) | 59.8 (50.9, 68.1) | 97.6 (93.2, 99.2) |
| DCP (≥7.5 ng/mL) | 40.5 (26.3, 56.5) | 62.0 (50.4, 72.4) | 52.5 (43.7, 61.2) | 93.6 (87.9, 96.7) |
| AFP (≥20 ng/mL) + DCP (≥7.5 ng/mL) | 67.6 (51.5, 80.4) | 80.3 (72.4, 86.4) | 76.2 (67.9, 82.3) | 91.2 (84.9, 95.0) |
Cutoff value corresponds to HelioLiver Test specificity of 91%.
As an exploratory endpoint, the sensitivity of the HelioLiver Test was compared with AFP and the GALAD score at the specificity determined for the HelioLiver Test (91.2%) (Table 2). At this standardized specificity, the sensitivity of the HelioLiver Test for HCC detection overall was 85.2% (95% CI 77.8%–90.4%), which was higher than AFP (cutoff = 12.1 ng/mL; 66.4%; 95% CI 57.6%–74.2%) and the GALAD score (cutoff = −1.2; 77.9%; 95% CI 69.8%–84.4%). The sensitivity of early‐stage HCC detection for the HelioLiver Test was 75.7% (95% CI 59.9%–86.7%), which remained higher than AFP (cutoff = 12.1 ng/mL; 59.5%; 95% CI 43.5%–73.4%) and the GALAD score (cutoff = −1.2; 70.3%; 95% CI 54.3%–82.5%). The sensitivity of the HelioLiver Test also remained higher than both AFP and the GALAD score at the remaining standardized specificities between 85% and 95% (Figure S3, Table S5).
The major underlying liver disease etiology in the ENCORE study was HBV, which is more prevalent in China compared with many other areas of the world. To gain insight surrounding this issue, a post hoc exploratory subgroup analysis was performed in subjects with non‐HBV etiologies (Table S6). AUROC of the HelioLiver Test (0.93; 95% CI 0.863–0.983) remained higher than AFP (0.913; 95% CI 0.852–0.974) and the GALAD score (0.901; 95% CI 0.825–0.977) within this non‐HBV subgroup (Figure S4). Additionally, the sensitivity of the HelioLiver Test (86.7%; 95% CI 70.4%–94.7%) was also higher than AFP (66.7%; 95% CI 48.8%–80.8%) and the GALAD score (80.0%; 95% CI 62.7%–90.5%) within this subgroup for all HCC (Table S7). These results suggest that the performance of the HelioLiver Test is etiology agnostic.
DISCUSSION
In this phase 2 cancer detection biomarker study as defined by the NCI Early Detection Research Network,[ 27 ] we confirmed that the HelioLiver Test demonstrated superior performance in detecting early‐stage (AJCC stage I and II) HCC compared with clinically available tests (AFP, AFP‐L3%, and DCP), the combination of AFP and DCP,[ 23 ] and the GALAD score.
The current standard of care for HCC surveillance is ultrasound either with or without AFP. A recent meta‐analysis of 32 separate studies (consisting of 13,367 patients) indicated ultrasound alone has a sensitivity of 84% (95% CI 76%–92%) for HCC of any stage and 47% (95% CI 33%–61%) for early‐stage HCC.[ 9 ] These relatively wide CIs for ultrasound sensitivity are indicative of the variability of ultrasound performance in different clinical settings. This variability is due in part to differences in the skill and training of the ultrasound operator, the quality of the instrument, and confounding factors that reduce ultrasound sensitivity, such as obesity and underlying liver disease.[ 28 ] Interestingly, many of these challenges posed to ultrasound as an HCC surveillance tool may be overcome by the development of a sensitive and easy‐to‐use blood test. Further subgroup analysis of studies comparing the performance of ultrasound with or without AFP indicated that including AFP increases the sensitivity for early HCC lesions from 45% for ultrasound alone to 63% for the combination of ultrasound and AFP.[ 9 ] However, the specificity is reduced from 92% to 84%.
Various meta‐analyses have calculated the pooled sensitivity and specificity for the individual protein tumor markers evaluated in this study to be 61% and 86% for AFP,[ 29 ] 56% and 90% for AFP‐L3%,[ 30 ] and 69% and 88% for DCP,[ 31 ] respectively. The combination of these three markers as part of the GALAD score consistently outperforms any of these markers individually. Within separate, multicenter studies of subjects with HCC with different etiologies of liver disease, the GALAD score was calculated to have sensitivities from 68% to 92% and specificities from 88% to 95%.[ 13 , 14 , 32 ] However, the combination of GALAD and ultrasound was found to possess superior performance characteristics than either test alone, with a sensitivity of 95% and specificity of 91%.[ 32 ] In this study, the sensitivity of the HelioLiver Test was found to be superior to AFP, AFP‐L3%, and DCP individually, and to the combination of these markers as part of the GALAD score.
Of note, the HelioLiver Test, which incorporates components of the GALAD score, achieved improved performance over the GALAD score itself by additionally measuring cfDNA methylation patterns from blood. Similar attempts also showed promising improvement of the GALAD score’s performance by combining additional tests and examinations such as ultrasound and tumor glycomics biomarkers,[ 19 , 20 ] suggesting that this is a valid strategy to incorporate such biomarkers and/or other HCC surveillance modalities for clinical translation.
Various types of biomolecules have been evaluated as potential HCC biomarkers. These include (1) a proteomics approach that identified a panel of seven serum protein markers[ 33 ]; (2) the DNA methylation marker Sept9,[ 34 , 35 ] which has previously been evaluated for detection of colorectal cancers; (3) the combination of detecting both protein biomarkers and cfDNA mutations by using an NGS approach and protein markers[ 36 ]; and (4) the measurement of up to six cfDNA methylation sites by using a polymerase chain reaction–based approach in combination with one to three protein markers.[ 37 , 38 ] A common observation in these studies is that assays combining multiple types of analytes generally achieve superior performance compared with single‐analyte methods, while other specific parameters to maximize performance of cfDNA‐based assays remain elusive. A recent technical assessment in gastrointestinal cancers, including HCC, suggested that approximately at least 10 to 50 cfDNA methylation markers are needed to achieve robust and accurate distinction between cancer cases and control subjects. This finding is consistent to our foundational work[ 16 , 17 ] and the number of cfDNA methylation markers (77 CpG markers in 28 genes) incorporated into the HelioLiver Test (Figure 1). As previously mentioned, we found that several of the 28 genes in our cfDNA panel are involved in molecular pathways implicated in HCC pathogenesis, such as mitogen‐activated protein kinase signaling, interleukin‐17, toll‐like‐receptor‐mediated innate immunity, and T/B‐cell receptor signaling.[ 24 , 25 ] In contrast, only one (MLK1) of the 497 unselected genes[ 26 ] has been associated in molecular pathways implicated in HCC pathogenesis (Figure S1). This suggests that our marker selection based solely on their HCC detection performance indeed enriched broad HCC‐pathogenesis‐related pathways, which may not be sufficiently covered by small gene panels. Such knowledge of underlying HCC biology may lead to the use of multianalyte blood tests, such as the HelioLiver Test, as a companion biomarker test to guide therapeutic interventions targeting relevant pathways. Additionally, the cfDNA methylation model used in the HelioLiver Diagnostic Algorithm was trained with the Integrative Training Set, which consisted exclusively of specimens collected from U.S. patients (Figure 2). In this study, we found that the HelioLiver Test outperformed both the GALAD score and each of the three evaluated protein tumor markers individually within a study cohort consisting entirely of patients from China. This finding suggests that the cfDNA methylation markers incorporated into the HelioLiver Test are not strongly influenced by the race or ethnicity. Further comparisons within boarder patient populations will be needed to evaluate this hypothesis.
Studies support the survival benefit of semi‐annual HCC surveillance, but its real‐world use is only 24% in all clinical settings and below 9% in community clinics.[ 39 , 40 ] Among the several obstacles for implementation and use of the recommended HCC surveillance approach, the requirement of abdominal ultrasound is a major logistical barrier that compromises adherence to semi‐annual surveillance.[ 41 ] If a blood test, such as the HelioLiver Test, is found to have favorable performance characteristics when compared with ultrasound‐based HCC surveillance, then HCC surveillance using a blood test may prove to be a more accessible option for patients. Reducing logistical barriers to semi‐annual surveillance is anticipated to significantly improve adherence or even inspire more frequent monitoring of at‐risk patients.
Despite the promising performance of our test, there are several limitations inherent to our study design. First, all subjects with HCC were diagnosed before enrollment. HCC diagnosis of all study subjects occurred after suspicion of HCC by ultrasound and/or serum AFP within a surveillance setting, or in subjects previously presenting with symptoms of HCC, which are not generally assumed in a surveillance setting. Thus, it is not feasible to directly compare the performance of the experimental tests assessed in this study to the current standard‐care surveillance method of ultrasound with or without AFP for the detection of HCC, as this population is biased for subjects in whom HCC was detected by the current surveillance techniques. Second, some control subjects with chronic liver diseases were diagnosed as HCC‐free based only on ultrasound and AFP rather than with diagnostic imaging such as multiphasic magnetic resonance imaging (MRI) or computed tomography. Therefore, although unlikely, it is possible that some of the control subjects may have undiagnosed HCC. Third, surveillance tests such as the HelioLiver Test are expected to have the most significant impact on health outcomes when HCC is detected at an early stage, when curative treatment options are more likely. The enrollment of a greater number of subjects with early‐stage HCC in follow‐up case‐control studies or prospective studies will allow for a more robust analysis of test performance for subjects with early HCC. Finally, the underlying HCC etiology was biased toward HBV infection, which is representative of the patient population in China.[ 42 , 43 , 44 ] The limitations in the design of our study are common among recently published studies to evaluate the performance of novel blood tests for HCC.( 33 , 34 , 35 , 36 , 37 , 38 , 45 ) To address these limitations, the HelioLiver Test is being further evaluated as part of a currently ongoing phase 3 biomarker study, in which the performance of the HelioLiver Test will be directly compared with standard‐of‐care ultrasound using multiphasic MRI as the gold standard for HCC diagnosis (CLiMB trial; NCT03694600). Prospective trials that directly compare the performance characteristics of the current standard‐of‐care surveillance methods to these novel blood tests for HCC detection are essential for evaluating the true contribution of these potential surveillance tools.
The HelioLiver Test was found to have a superior sensitivity for HCC and a similar specificity when compared to both AFP alone and the GALAD score. Most importantly, the HelioLiver Test demonstrated a superior sensitivity for early‐stage (AJCC I and II) HCC when compared with either AFP testing alone or the GALAD score. The implementation of a blood test such as the HelioLiver Test will enable easy, flexible, noninvasive, and accurate HCC detection at early stages, and significantly improve treatment outcomes for a transformative reduction of HCC mortality.
CONFLICT OF INTEREST
Jianfeng Xu, Maxime A. Gallant, Dhruvajyoti Roy, and David Taggart are employees of Helio Health. Dan Liu is an employee of the Laboratory for Advanced Medicine. Diange Li is employed by Guangzhou Youze Biological Pharmaceutical Technology Company. Robert Gish consults for, advises for, is on the speakers’ bureau for, and received grants from Gilead. He consults for, advises for, and is on the speakers’ bureau for AbbVie, Bayer, Bristol‐Myers Squibb, Eisai, Intercept, and Salix. He consults for, advises for, and is on the data safety monitoring board for Arrowhead. He consults for, advises for, and owns stock in Eiger, Genlantis, and HepQuant. He consults for and advises for Abbott, Access Biologicals, Antios, Dova, Dynavax, Enyo, Forty‐Seven, Fujifilm/Wako, eStudySite, Genentech, Gerson Lehrman, HepaTX, Janssen, Helios, Lilly, Merck, Shionogi, and Viking. He consults for ADMA, AEC, Aligos, Arena, Arterys, Alexion, Altimmune, AprosTx, Cirina, Consumer Health Products Association, DiaSorin, DRG Abacus, DURECT, Echosens, Exelixis, IDLogiq, Intellia, Inotek, Iqvia, KannaLife, Laboratory for Advanced Medicine, Labyrinth Holdings, MedImmune, New Enterprise Associates, Ogilvy, Organovo, Patient Connect, ProdigY Biotech, Prometheus, Refuah Solutions, Regulus, Spring Bank, and Trimaran. He advises for Biocollections, Prodigy, and Quest. He owns stock in RiboSciences, CoCrystal, and AngioCrine. Ghassan Abou‐Alfa serves as a consultant for or serves on scientific advisory boards for Agios, AstraZeneca, Autem Medical, Bayer, Beigene, Berry Genomics, Celgene, Eisai, Flatiron Health, Genoscience, Gilead, Incyte, Ipsen, LAM, Lilly, Merck Serono, Minapharm, QED, RedHill Biopharma, Roche/Genentech, SillaJen, TheraBionic, twoXAR, and Vector Health. He receives travel/accommodation funding from Polaris. He receives research funding (to institution) from Agios, AstraZeneca, Bayer, Bristol Myers Squibb, CASI, Exelixis, Incyte, Polaris, Puma Biotechnology, and QED outside the submitted work. Mindie H. Nguyen was previously a consultant for the Laboratory for Advanced Medicine and has received research support from Pfizer, Enanta, Gilead, Exact Sciences, Vir Biotech, Helio Health, National Cancer Institute, Glycotest, and B.K. Kee Foundation. Mindie Nguyen also received personal fees as a consultant and/or advisory board member for Intercept, Exact Sciences, Gilead, GSK, Eli Lilly, and Janssen. Richard Van Etten serves as an advisory board member for Helio Health. Yujin Hoshida serves as an advisory board member for Helio Health and founding shareholder for Alentis Therapeutics and received a research funding from Morphic Therapeutics. Wei Li is a consultant for Helio Health and ChosenMed.
TRIAL REGISTRATION
ClinicalTrials.gov identifier: NCT05059665.
Supporting information
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients who generously participated in the clinical trial and the principal investigators and institutions who oversaw their enrollments. They would also like to acknowledge Dr. Shivani Mahajan for performing the logistic regression analysis, to assess confounding variables for the HelioLiver Test.
Lin N, Lin Y, Xu J, Liu D, Li D, Meng H, et al. A multi‐analyte cell‐free DNA–based blood test for early detection of hepatocellular carcinoma. Hepatol Commun. 2022;6:1753–1763. 10.1002/hep4.1918
Nan Lin, Yongping Lin, and Jianfeng Xu contributed equally to this work.
Funding information
Helio Health and the Laboratory for Advanced Medicine
Contributor Information
David J. Taggart, Email: davidt@heliohealth.com.
Yujin Hoshida, Email: yujin.hoshida@utsouthwestern.edu.
Wei Li, Email: wei.li@uci.edu.
REFERENCES
- 1. Mokdad AH, Dwyer‐Lindgren L, Fitzmaurice C, Stubbs RW, Bertozzi‐Villa A, Morozoff C, et al. Trends and patterns of disparities in cancer mortality among US counties, 1980–2014. JAMA. 2017;317:388–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 3. Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34:1787–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- 5. Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–80. [DOI] [PubMed] [Google Scholar]
- 7. European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. [DOI] [PubMed] [Google Scholar]
- 8. Omata M, Cheng A‐L, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia‐Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017;11:317–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta‐analysis. Gastroenterology. 2018;154:e1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, et al. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45:169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wong LL, Reyes RJ, Kwee SA, Hernandez BY, Kalathil SC, Tsai NC. Pitfalls in surveillance for hepatocellular carcinoma: How successful is it in the real world? Clin Mol Hepatol. 2017;23:239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144–53. [DOI] [PubMed] [Google Scholar]
- 13. Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, et al. Role of the GALAD and BALAD‐2 serologic models in diagnosis of hepatocellular carcinoma and prediction of survival in patients. Clin Gastroenterol Hepatol. 2016;14:e876. [DOI] [PubMed] [Google Scholar]
- 14. Best J, Bechmann LP, Sowa J‐P, Sydor S, Dechêne A, Pflanz K, et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2020;18:e724. [DOI] [PubMed] [Google Scholar]
- 15. Tran NH, Kisiel J, Roberts LR. Using cell‐free DNA for HCC surveillance and prognosis. JHEP Rep. 2021;3:100304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hao X, Luo H, Krawczyk M, Wei W, Wang W, Wang J, et al. DNA methylation markers for diagnosis and prognosis of common cancers. Proc Natl Acad Sci U S A. 2017;114:7414–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu R‐H, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, et al. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155–61. [DOI] [PubMed] [Google Scholar]
- 18. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, Zhou W, et al. Guidelines for the diagnosis and treatment of hepatocellular carcinoma. Liver Cancer. 2020;9:682–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xi Y, Li W. BSMAP: whole genome bisulfite sequence MAPping program. BMC Bioinformatics. 2009;10:232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, et al. The Sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Quinlan AR, Hall IM. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gupta S, Bent S, Kohlwes J. Test characteristics of alpha‐fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. [DOI] [PubMed] [Google Scholar]
- 23. Ertle JM, Heider D, Wichert M, Keller B, Kueper R, Hilgard P, et al. A combination of alpha‐fetoprotein and des‐gamma‐carboxy prothrombin is superior in detection of hepatocellular carcinoma. Digestion. 2013;87:121–31. [DOI] [PubMed] [Google Scholar]
- 24. Li N, Yamamoto G, Fuji H, Kisseleva T. Interleukin‐17 in liver disease pathogenesis. Semin Liver Dis. 2021;41:507–15. [DOI] [PubMed] [Google Scholar]
- 25. Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21:541–57. [DOI] [PubMed] [Google Scholar]
- 26. Liu Z, Sun J, Li C, Xu L, Liu J. MKL1 regulates hepatocellular carcinoma cell proliferation, migration and apoptosis via the COMPASS complex and NF‐kappaB signaling. BMC Cancer. 2021;21:1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pepe MS, Etzioni R, Feng Z, Potter JD, Thompson ML, Thornquist M, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. [DOI] [PubMed] [Google Scholar]
- 28. Sparchez Z, Craciun R, Caraiani C, Horhat A, Nenu I, Procopet B, et al. Ultrasound or sectional imaging techniques as screening tools for hepatocellular carcinoma: fall forward or move forward? J Clin Med. 2021;10:903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Chen G, Zhang P, Zhang J, Li X, Gan D, et al. The threshold of alpha‐fetoprotein (AFP) for the diagnosis of hepatocellular carcinoma: a systematic review and meta‐analysis. PLoS One. 2020;15:e0228857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen S, Li J, Tan X, Xu QI, Mo Y, Qin H, et al. Clinical role of combining alpha‐fetoprotein and lens culinaris agglutinin‐reactive fraction of alpha‐fetoprotein for hepatocellular carcinoma: evidence from literature and an original study. J Clin Lab Anal. 2020;34:e23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen H, Chen S, Li S, Chen Z, Zhu X, Dai M, et al. Combining des‐gamma‐carboxyprothrombin and alpha‐fetoprotein for hepatocellular carcinoma diagnosing: an update meta‐analysis and validation study. Oncotarget. 2017;8:90390–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, et al. GALAD score for hepatocellular carcinoma detection in comparison with liver ultrasound and proposal of GALADUS score. Cancer Epidemiol Biomarkers Prev. 2019;28:531–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang S, Liu Y, Chen J, Shu H, Shen S, Li Y, et al. Autoantibody signature in hepatocellular carcinoma using seromics. J Hematol Oncol. 2020;13:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kotoh Y, Suehiro Y, Saeki I, Hoshida T, Maeda M, Iwamoto T, et al. Novel liquid biopsy test based on a sensitive methylated SEPT9 assay for diagnosing hepatocellular carcinoma. Hepatol Commun. 2020;4:461–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oussalah A, Rischer S, Bensenane M, Conroy G, Filhine‐Tresarrieu P, Debard R, et al. Plasma mSEPT9: a novel circulating cell‐free DNA‐based epigenetic biomarker to diagnose hepatocellular carcinoma. EBioMedicine. 2018;30:138–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, et al. Detection of early‐stage hepatocellular carcinoma in asymptomatic HBsAg‐seropositive individuals by liquid biopsy. Proc Natl Acad Sci U S A. 2019;116:6308–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chalasani NP, Porter K, Bhattacharya A, Book AJ, Neis BM, Xiong KM, et al. Validation of a novel multitarget blood test shows high sensitivity to detect early stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021;20:173–82. [DOI] [PubMed] [Google Scholar]
- 38. Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, Edwards VD, Roberts LR, et al. A novel blood‐based panel of methylated DNA and protein markers for detection of early‐stage hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2021;19:2597–605. [DOI] [PubMed] [Google Scholar]
- 39. Singal AG, Lok AS, Feng Z, Kanwal F, Parikh ND. Conceptual model for the hepatocellular carcinoma screening continuum: current status and research agenda. Clin Gastroenterol Hepatol. 2022;20:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wolf E, Rich NE, Marrero JA, Parikh ND, Singal AG. Use of hepatocellular carcinoma surveillance in patients with cirrhosis: a systematic review and meta‐analysis. Hepatology. 2021;73:713–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Farvardin S, Patel J, Khambaty M, Yerokun OA, Mok H, Tiro JA, et al. Patient‐reported barriers are associated with lower hepatocellular carcinoma surveillance rates in patients with cirrhosis. Hepatology. 2017;65:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen W. Cancer statistics: updated cancer burden in China. Chin J Cancer Res. 2015;27:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lin X, Robinson NJ, Thursz M, Rosenberg DM, Weild A, Pimenta JM, et al. Chronic hepatitis B virus infection in the Asia‐Pacific region and Africa: review of disease progression. J Gastroenterol Hepatol. 2005;20:833–43. [DOI] [PubMed] [Google Scholar]
- 44. Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65–73. [DOI] [PubMed] [Google Scholar]
- 45. Kisiel JB, Dukek BA, V.S.R. Kanipakam R, Ghoz HM, Yab TC, Berger CK, et al. Hepatocellular carcinoma detection by plasma methylated DNA: discovery, phase I pilot, and phase II clinical validation. Hepatology. 2019;69:1180–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


