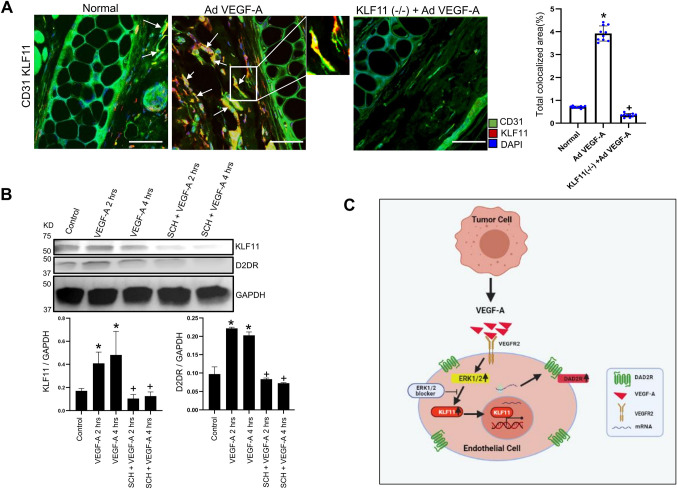
Fig. 5.
VEGF-A-induced upregulation of KLF11 expression in endothelial cells is abrogated by blocking the ERK1/2 pathway. (A) AdVEGF-A injection into the ears leads to a significantly high angiogenic response (CD31) along with higher colocalization (arrows) of KLF11 (red) in the endothelial cells (CD31; green) of wild-type C57/BL6 mice but not in the endothelial cells of ear blood vessels of the endothelial cell-specific KLF11-knockout mice (n=10 for each group). Scale bars: 40 µm. Right, ten fields of view were randomly chosen and analyzed. Data are expressed as the mean±s.e.m. *P<0.05 for KLF11(−/−) injected with AdVEGF-A compared with the normal; +P<0.05 for KLF11−/− compared with wild type C57/BL6 mice, both injected with AdVEGF-A (one-way ANOVA with Bonferroni step down adjustment). (B) Western blot demonstrating that VEGF-A significantly upregulated KLF11 as well as DAD2 receptor (D2DR) expression in endothelial cells, and pretreatment of these cells with selective ERK1/2 blocker SCH772984 (SCH), 1 µM, abolished the action of VEGF-A. The fold changes of KLF11 and DAD2 receptors normalized to GAPDH from different experiments were averaged. Data are expressed as mean±s.e.m. and is representative of three independent experiments with similar results. *P<0.005 for VEGF-A versus vehicle control; +P<0.005 for SCH+VEGF-A versus VEGF-A (one-way ANOVA with Bonferroni step down adjustment). (C) A schematic diagram showing that VEGF-A secreted by tumor cells stimulates the expression of dopamine D2 receptors (DAD2R) in the endothelial cells by activating the ERK1/2 signaling cascade and upregulating KLF11 expression in these cells. This panel was created with BioRender.com.