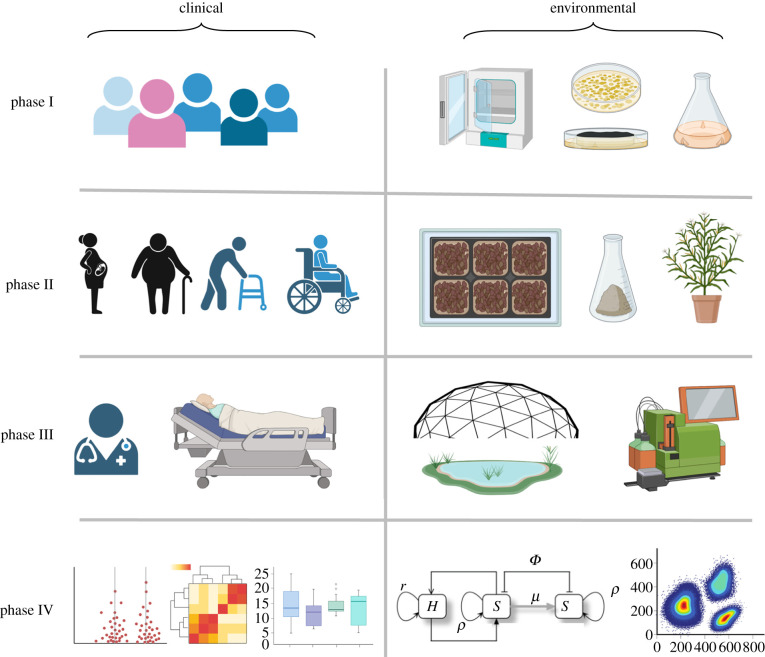
Figure 5.
Comparison of standardized phases of clinical trials that precede approval of a new drug or treatment versus possible stages of testing efficacy and safety of biologicals for environmental release. In the human health-related roadmap, phase I studies application to a small group of people just to evaluate safety and possible side effects. Phase II studies both the efficacy and possible adverse effects of the treatment in a larger group of more diverse individuals. The critical phase III extends efficacy/safety studies to still larger population of patients in different regions and countries. If the outcome is positive, it may suffice to be approved. Phase IV is a post-adoption surveillance stage that studies effects that were not seen in earlier trials. Equivalent stages for environmental applications could involve the following. Phase I, testing the biological in laboratory-scale microcosm with a limited number of components for stability, expression of the trait of interest and impact on structure of a pre-existing community. Phase II, same in mesocosms with more actors and a larger variety of physico-chemical conditions. Phase III, scaling-up to an ecotron-size with a complete ecosystem including soil and water matrices along with plants, insects and a controlled, contained atmosphere—along with instruments to quantify relevant parameters. Phase IV involves post-adoption analyses and modelling of the new scenario and surveillance of emergent phenomena.