Abstract
Purpose:
We report the 5-year follow-up findings of a randomized, open-label, phase II trial of lobaplatin-based neoadjuvant chemotherapy plus adjuvant therapy for triple-negative breast cancer (TNBC).
Patients and methods:
This study included patients aged ⩾18 years with untreated, operable stage I–III TNBC and an Eastern Cooperative Oncology Group performance status of 0 or 1. One group of patients (TE group, n = 99) received four cycles of docetaxel (T, 75 mg/m²) plus epirubicin (E, 80 mg/m²) every 3 weeks, and another group (TEL group, n = 101) received the same treatment with the addition of lobaplatin (L, 30 mg/m2). Two cycles of the corresponding treatments were administered after surgery in both groups. The primary endpoints were total pathological complete response (tpCR) rate and overall response rate (ORR), and the secondary endpoints were disease-free survival, overall survival, and long-term safety. This trial is registered with the Chinese Clinical Trial Registry (ChiCTR-TRC-14005019).
Results:
The median follow-up was 48.2 months (interquartile range: 31.1–60.0). The tpCR rate was 41.4% and 17.8% in the TEL group and TE group, respectively (p < 0.001). The HR for comparison of DFS between the TEL group and TE group was 0.44 (95% CI: 0.21–0.90, P p = 0.028). The addition of lobaplatin resulted in an HR of 0.44 (95% CI: 0.18–1.02, P = 0.061) for the difference in OS between the two groups. The ORR, which included complete response and partial response, was 92.9% in the TEL group and 74.3% in the TE group (p = 0.001). The TEL group patients were more likely to develop grade III–IV anemia and thrombocytopenia. No lobaplatin-related deaths or increased risk of long-term toxicity was observed.
Conclusion:
Neoadjuvant lobaplatin therapy can improve the tpCR and ORR rates of TNBC with tolerable side effects and have a tendency to improve the long-term survival.
Keywords: neoadjuvant chemotherapy, lobaplatin, platinum, prognosis, triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer that accounts for 12%–17% of all breast cancers, and lacks the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).1,2 Due to a lack of targeted therapies for its treatment, TNBC has a greater metastatic potential, a higher relapse rate, poorer clinical outcomes, and lower survival rates than other subtypes of breast cancer. In patients who receive systemic treatments and develop metastatic disease, the median overall survival (OS) is only 13–18 months. 3
Neoadjuvant chemotherapy with anthracycline and/or taxane is the standard treatment for early stage or locally advanced TNBC. Approximately one-third of patients with stage II to III TNBC treated with neoadjuvant chemotherapy achieve total pathologic complete response (tpCR). Achieving tpCR (defined as no invasive cancer in the breast and axilla, ypT0/is ypN0) at the time of surgery is associated with improved survival in TNBC.4 –6 Platinum-based neoadjuvant chemotherapy combined with docetaxel (T) and epirubicin (E) is currently being evaluated for the treatment of TNBC, and some randomized clinical trials have confirmed that platinum-based neoadjuvant chemotherapy significantly increases the pCR rates in TNBC.7 –11
Lobaplatin (1,2-diammino-methyl-cyclobutane-platinum (II)-lactate) is a third-generation platinum drug that shows robust antitumor activity with lower toxicity than that of other platinum-based compounds (such as cisplatin and carboplatin) and has been approved in China for the treatment of several malignancies.12 –16 The mechanism of action of lobaplatin is similar to that of other platinum-based compounds and involves the formation of DNA adducts and cell apoptosis. 17 In a phase II study on relapsed ovarian cancer, lobaplatin chemotherapy showed promising clinical activity, 18 and in another phase II study on recurrent and metastatic nasopharyngeal carcinoma, lobaplatin-based and docetaxel-based chemotherapy demonstrated acceptable efficacy and safety. 19 Despite these promising findings, there is limited evidence regarding the efficacy and safety of neoadjuvant chemotherapy with concomitant lobaplatin use in standard chemotherapy for TNBC. Therefore, this clinical trial was conducted to assess the efficacy and safety of neoadjuvant chemotherapy with lobaplatin in untreated and operable TNBC patients. We have previously published early evidence that the addition of neoadjuvant chemotherapy with lobaplatin to the TE regimen for TNBC significantly improved the tpCR and overall response rate (ORR) rates with tolerable side effects. 20 In this study, we present an updated analysis with the 5-year follow-up findings of this clinical trial, including long-term survival and toxicity.
Materials and methods
Study design and participants
This study was a prospective open-label randomized clinical trial conducted at the First Affiliated Hospital of the Third Military Medical University (Army Medical University). The presented data pertain to the updated results from the ongoing trial. The interim analysis from this study has been summarized in a previous report. 20 Based on the selection criteria, the trial included female patients aged 18–70 years with newly diagnosed or previously untreated, operable, clinical stage I to III TNBC. The other inclusion criteria were Eastern Cooperative Oncology Group performance status 0 or 1; normal hepatic, renal, and cardiac function; and normal blood counts. In all the included cases, the triple-negative status was histologically confirmed based on <10% ER and PR expression, determined by local immunohistochemical (IHC) analysis, and absence of HER2 (IHC score 0 to 1+, or IHC score 2+ with no amplification according to the results of in situ hybridization).21,22 The key exclusion criteria were the presence of distant metastases, previous neoadjuvant chemotherapy, clinically significant cardiac disease, presence of peripheral neuropathy, current pregnancy and child-bearing potential, any other physical or psychological condition that could affect the patient’s conduct in the study, and participation in any other clinical trial.
Randomization and blinding
Eligible patients were randomly assigned to receive docetaxel and epirubicin (TE) with or without additional lobaplatin (L) treatment at a 1:1 ratio. The randomization sequence was generated using Research Randomizer (www.randomizer.org). This study’s statistical team was blinded to the treatment assignments; however, both patients and investigators were aware of the treatment assignment (open label).
Procedures
All patients recruited in this study underwent baseline measurements, including physical examination, laboratory tests (complete blood count and serum chemistry), and imaging studies (e.g. mammogram, breast ultrasound and magnetic resonance imaging, computed tomography, abdominal ultrasound, and bone scans) to exclude metastatic disease. Clinically positive axillae were confirmed by biopsy.
The lobaplatin group received four cycles of docetaxel, epirubicin, and lobaplatin before surgery (TEL group: docetaxel 75 mg/m2 as a 1-h intravenous infusion on the first day of every 3-week cycle with concurrent epirubicin at a dose of 80 mg/m2 and lobaplatin at a dose of 30 mg/m2). The control group (TE group) received the same regimen, with the exception of lobaplatin. The patients received two cycles of the same treatments after surgery, unless disease recurrence or intolerable adverse events occurred, or they withdrew their consent. No crossover was permitted in the study. Drug compliance was monitored during the trial. Toxicities were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0).
Outcomes
The primary endpoints were tpCR rate and ORR. ORR was defined as the proportion of patients who achieved CR or partial response (PR). Tumor assessment was evaluated using Response Evaluation Criteria in Solid Tumors criteria version 1.1 for the objective response rate. Tumor assessment by ultrasound imaging was performed after the four treatment cycles and before surgery. The secondary endpoints were disease-free survival (DFS), OS, and long-term safety. DFS was defined as the period from randomization to the first radiographic record of disease progression. OS was defined as the time from randomization to death, and a final analysis will be performed after 5 years of survival data have been collected. In the current analysis, the measurement of long-term safety is focused on adverse events and serious adverse events, which were recorded and graded according to the standard criteria.
Statistical analysis
The planned sample size of TNBC patients was 200 in this study. According to the results of the GeparSixto trial reported at the annual meeting of the American Society of Clinical Oncology in 2013, the tpCR associated with paclitaxel and adriamycin treatment was 37.9% and the tpCR associated with paclitaxel, adriamycin, and carboplatin treatment was 58.7%. We used these results to estimate the sample size for our study, as the design of the GeparSixto trial was similar to that of our trial at that time. With the significance threshold (α) set at 0.05 and the statistical power (1 − β) set at 0.8, it was estimated that each group would require 90 participants to achieve statistically significant inter-group differences. Furthermore, 10% of the patients were expected to withdraw during the study period. Hence, ultimately, the sample size of each group was determined to be 100, based on which it was decided that a total of 200 participants would be recruited. The OS analyses were based on the intention-to-treat method, so the present study is prone to achieve negative result. DFS and OS were compared between the two groups with a log-rank test. The Kaplan–Meier method was used to estimate the hazard ratios (HRs) and 95% confidential intervals (CIs) were estimated by a Cox proportional hazards model. Safety analyses were based on the treatment that was received. Statistical analyses were performed with the IBM SPSS Statistics software, version 26.
Results
Patient characteristics
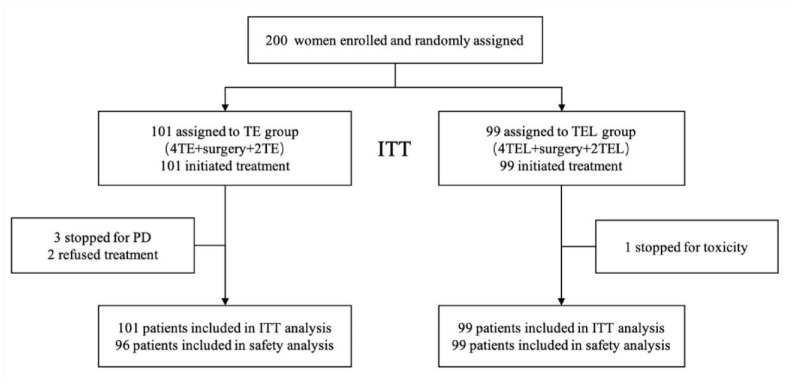
Between 2 January 2014 and 19 August 2019, a total of 200 eligible women from the First Affiliated Hospital of Third Military Medical University (Army Medical University), Chongqing, China, were enrolled in this study and randomly assigned to the TE group (n = 101) or the TEL group (n = 99; Figure 1). Three patients discontinued treatment due to progressive disease (PD); two patients refused treatment; and one patient discontinued treatment due to drug toxicity. A total of 194 patients completed all the treatment cycles. Table 1 lists the baseline characteristics of the two groups (the data are similar to the previously published results). 20
Figure 1.
Selection process of the trial participants.
CONSORT flow chart showing enrollment of 200 participants, including 99 participants in the intervention group and 101 in the usual treatment group, who completed the study and were included in the outcome analyses.
Table 1.
Baseline characteristics.
| TE group (n = 101) | TEL group (n = 99) | |
|---|---|---|
| Age, years | ||
| <40 | 13 (13%) | 16 (16%) |
| 40–59 | 77 (76%) | 76 (77%) |
| ⩾60 | 11 (11%) | 7 (7%) |
| BMI | ||
| Underweight | 2 (2%) | 2 (2%) |
| Normal | 61 (66%) | 65 (66%) |
| Overweight | 25 (27%) | 24 (24%) |
| Obese | 4 (4%) | 8 (8%) |
| Clinical stage | ||
| I | 18 (18%) | 16 (16%) |
| II | 64 (63%) | 62 (63%) |
| III | 19 (19%) | 21 (21%) |
| N stage | ||
| 0 | 41 (41%) | 53 (54%) |
| 1 | 38 (38%) | 25 (25%) |
| 2 | 12 (12%) | 7 (7%) |
| 3 | 10 (10%) | 14 (14%) |
| T stage | ||
| T1 | 32 (32%) | 36 (36%) |
| T2 | 60 (60%) | 54 (55%) |
| T3 | 9 (9%) | 9 (9%) |
| T4 | 0 (0%) | 0 (0%) |
Data are median (interquartile range) or n (%).
BMI, body mass index; TE, docetaxel and epirubicin, TEL, docetaxel, epirubicin, and lobaplatin.
Treatment response
The tpCR rate of the patients who received the TEL regimen was 41.4% (41/99), while it was lower at 17.8% (18/101) in patients who received the TE regimen [relative risk (RR) = 1.93; 95% CI: 1.28–2.91; p < 0.001; Figure 2). As summarized in Supplemental Table S1, in the TEL group, 44 patients achieved CR, 48 achieved PR, and 7 developed stable disease (SD), and there were no cases of PD. The ORR, which included CR and PR, of the TEL group was 92.9% (92/99). In the TE group, 31 patients achieved CR, 44 patients achieved PR, 23 patients developed SD, and 3 patients experienced PD. The ORR of the TE group was 74.3% (75/101). The ORR of the TEL group was significantly higher than that of the TE group (Supplemental Figure S1; RR = 1.75, 95% CI: 1.37–2.24, p = 0.001).
Figure 2.
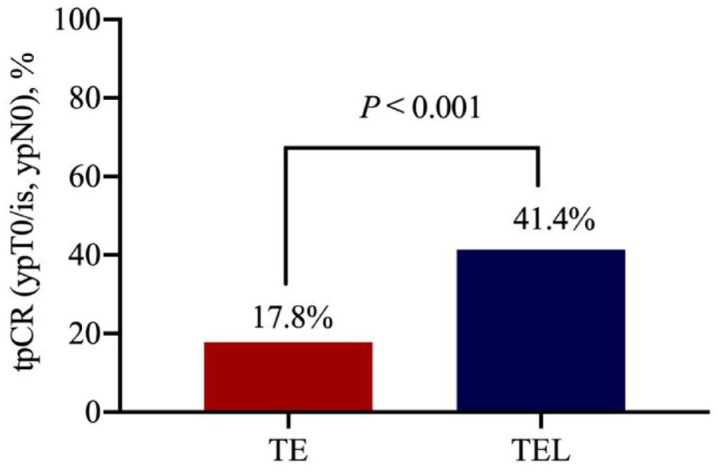
Assessment of pathologic CR.
tpCR of TNBC patients after four cycles of neoadjuvant chemotherapy.
CR, complete response; TE, docetaxel and epirubicin; TEL, docetaxel, epirubicin, and lobaplatin; TNBC, triple-negative breast cancer; tpCR, total pathologic CR.
Survival
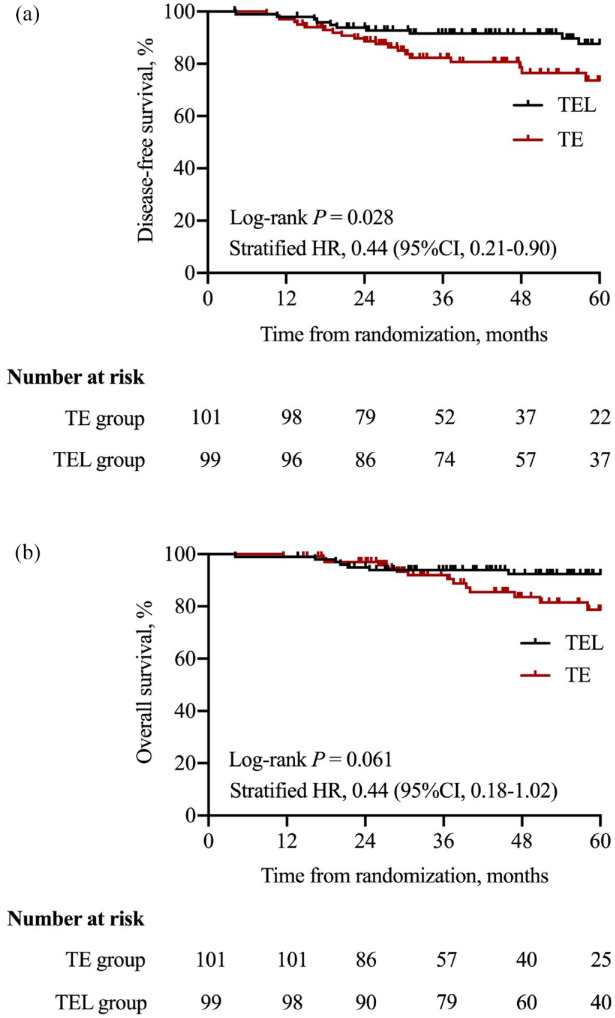
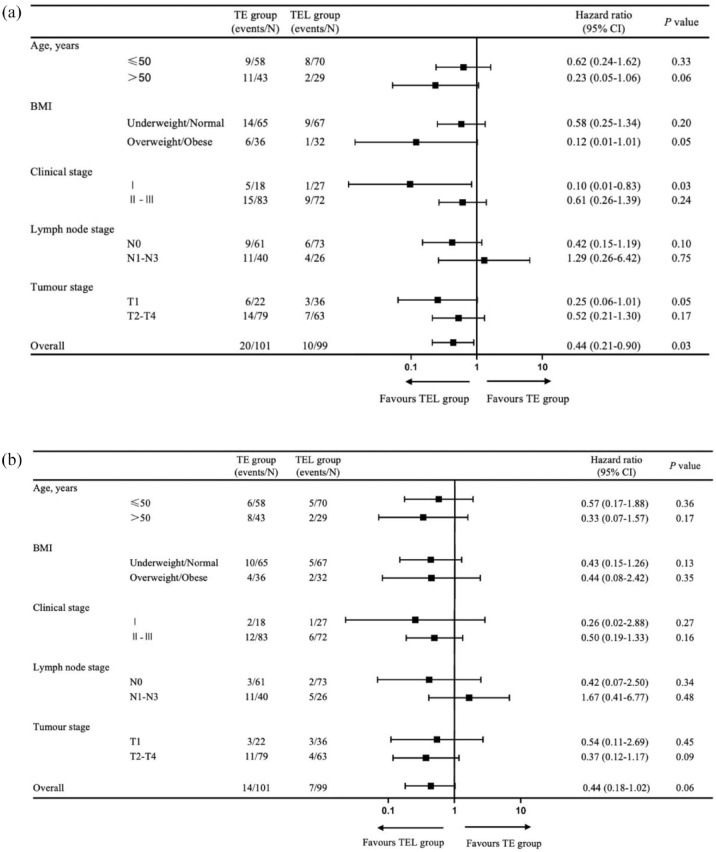
In this updated analysis, the median duration of the follow-up period was 48.2 months (interquartile range: 31.1–60.0). At the end of the study, 10 (10.1%) patients in the TEL group and 20 (19.8%) patients in the TE group had invasive DFS events. The HR for comparison of DFS between the TEL group and TE group was 0.44 (95% CI: 0.21–0.90, p = 0.028) (Figure 3(a)). Subgroup analysis adjusted for the stratification factors showed similar results (Figure 4(a)).
Figure 3.
Comparison of survival outcomes between treatment groups: (a) DFS and (b) OS.
DFS, disease-free survival; HR, hazard ratio; OS, overall survival; TE, docetaxel–epirubicin; TEL, docetaxel–epirubicin–lobaplatin.
Figure 4.
Forest plot for (a) DFS and (b) OS.
DFS, disease-free survival; OS, overall survival.
A total of 21 patients died and all the deaths were disease related. A total of seven deaths occurred in the TEL group compared to 14 deaths in the TE group. The addition of lobaplatin resulted in an HR of 0.44 (95% CI: 0.18–1.02, p = 0.061) (Figure 3(b)) for the difference in OS between the two groups. Subgroup analysis of OS adjusted for the stratification factors revealed similar results (Figure 4(b)).
Toxicity
All the patients included in this clinical trial underwent blood tests before and after each chemotherapy cycle or as required for long-term toxicity assessment. The most common hematological adverse events in the TE group were anemia (84/96, 87.5%) and leukopenia (66/96, 68.8%), which were also the most common adverse events in the TEL group (anemia: 91/99, 91.9%; leukopenia: 83/99, 83.8%) (Table 2). Serious hematological toxicities were defined as any unexpected grade III–IV toxicity. Compared with the TE group, patients in the TEL group were more likely to develop grade III–IV anemia (52.5.0% versus 12.5%, p < 0.001) and thrombocytopenia (35.4% versus 3.1%, p < 0.001) (Table 2). Grade III–IV treatment-related non-hematologic toxicities included vomiting (6/98 and 2/96 in the TEL and TE groups, respectively) and diarrhea (5/98 and 3/96 in the TEL and TE groups, respectively). There were two cases of subcutaneous hemorrhage and two cases of phlebitis in the TEL group (Supplemental Table S2). No substantial toxicity was observed in the livers, kidneys, or neurons.
Table 2.
Hematological toxicities.
| TE group (96) N (%) | TEL group (99) N (%) | p value | |
|---|---|---|---|
| Leukopenia | 0.010 | ||
| I–II | 45 (47) | 50 (51) | |
| III–IV | 21 (22) | 33 (33) | |
| Neutropenia | 0.003 | ||
| I–II | 35 (36) | 33 (33) | |
| III–IV | 20 (21) | 39 (39) | |
| Anemia | <0.001 | ||
| I–II | 72 (75) | 39 (39) | |
| III–IV | 12 (12) | 52 (53) | |
| Thrombocytopenia | <0.001 | ||
| I–II | 13 (14) | 17 (17) | |
| III–IV | 3 (3) | 35 (35) | |
TE, docetaxel and epirubicin; TEL docetaxel, epirubicin, and lobaplatin.
Discussion
Neoadjuvant chemotherapy is a standard care modality for locally advanced and operable TNBC. A major advantage of this approach is the ability to pre-emptively predict survival according to the presence or absence of tpCR at the time of surgery and to tailor adjuvant therapy accordingly. In this clinical trial, the regimen was administered in part before and in part after surgery, according to clinical practice guidelines for breast cancer by the Chinese Anti-Cancer Association, version 2011. It should be noted that these guidelines differ from the current global standards of care. With the regimen used in this trial, clinical response to neoadjuvant chemotherapy can be assessed; however, some patients who could have achieved tpCR may not have achieved it as the number of cycles of neoadjuvant chemotherapy were not enough. This might have led to a decrease in the tpCR rates. Nonetheless, in accordance with the previous findings from the interim analysis, 20 the results of the mature analysis in this study also indicated that the tpCR rate in the TEL group was significantly higher than that in the TE group. However, the occurrence of multiple adverse events, including anemia, leukopenia, thrombocytopenia, and neutropenia, was more frequent in the TEL group than in the TE group.
Previous studies have shown that platinum-based neoadjuvant chemotherapy, especially the addition of carboplatin, is associated with high pCR rates, DFS, and OS in TNBC patients.23 –26 Regimens containing platinum reagents have been found to be highly effective for pCR. 25 However, the efficiency of lobaplatin for the treatment of TNBC is largely unknown, although other studies have shown that it is beneficial for the treatment of non-small-cell lung cancer, colorectal cancer, nasopharyngeal carcinoma, and metastatic breast cancer.16,27 –29 Therefore, the present findings make an important contribution to what is known about the therapeutic effects of lobaplatin.
Recent evidence has also shown that immunotherapy is a promising treatment strategy for neoadjuvant therapy of TNBC. 30 For example, Schmid et al. reported that the addition of pembrolizumab led to a significant increase of 13.6% in pCR compared to the control group. 31 Our study found that the addition of lobaplatin had a comparable effect size to immunotherapy. Based on these findings, combining our regimen with monoclonal antibodies, such as programmed death ligand-1, warrants investigation, as it could further improve the prognosis of TNBC patients. In addition to lobaplatin, capecitabine and olaparib are also optional drugs for adjuvant chemotherapy of TNBC. Since the evidence of lobaplatin’s effect on TNBC has only recently emerged, in the future, there is a need to compare the efficacy and safety of lobaplatin to that of capecitabine or other options, and to examine the performance of lobaplatin when used in combination with these drugs.
To the best of our knowledge, our trail is the first to focus on the effects of lobaplatin-based neoadjuvant chemotherapy on the prognosis of patients with TNBC. Our pilot study showed that patients who received lobaplatin-based neoadjuvant chemotherapy had a higher tpCR rate than those who received the standard treatment. 20 In the present study, we show that lobaplatin-based neoadjuvant chemotherapy also has long-term advantages in terms of improving DFS in TNBC patients. The mechanism of lobaplatin’s anti-neoplastic effect may be attributed to JNK (c-Jun N-terminal) phosphorylation, ROS activation (reactive oxygen species), cell pyroptosis, and apoptosis.32,33 However, the specific mechanism by which lobaplatin facilitates TNBC elimination warrants further investigation.
Some studies have indicated that lobaplatin can induce grade III–IV hematologic toxicity, including neutropenia and thrombocytopenia.19,34 Our study presents similar results as those of previous publications. 35 The proportion of patients with grade III–IV leukopenia, neutropenia, anemia, and thrombocytopenia was higher in the TEL group than in the control (TE) group. These findings indicate that hematologic toxicity may be a concern for lobaplatin therapy.
One limitation of this study is that the subtype of TNBC was not determined. It has been reported that the basal subtype is associated with worse recurrence-free survival and OS in patients with TNBC, but is more sensitive to platinum. Therefore, there could have been subtype-dependent differences in the response to therapy that were missed. In addition, in the future, pharmacogenomic studies of genetic polymorphisms, including BRCA mutation status, can help identify specific subsets of patients who could potentially benefit more from lobaplatin-based systemic intervention.
In summary, lobaplatin-based neoadjuvant chemotherapy has high activity and prolonged DFS in TNBC, but may lead to hematologic toxicity in a certain portion of patients. The current study was limited by its small sample size, as well as the lack of genomic data. Therefore, a future multi-center study with a large sample size is required to validate the current findings.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221107111 for Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial by Wenting Yan, Xiujuan Wu, Shushu Wang, Cheng He, Ling Zhong, Peng Tang, Lin Ren, Ting Zhang, Xiaowei Qi and Yi Zhang in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to express their sincere thanks to the volunteers of the study.
Footnotes
Ethics approval and consent to participate: This study was approved by the ethics committee of the First Affiliated Hospital of the Third Military Medical University (Army Medical University) (Approval no. KY-2014-15) and was registered in the Chinese Clinical Trial Registry (http://www.chictr.org.cn/) on 29 May 2014 (Trial no.ChiCTR-TRC-14005019). The trial was performed according to the Good Clinical Practice guidelines and the tenets of the Declaration of Helsinki. All the patients provided their written informed consent prior to the start of the study.
Consent for publication: Not applicable.
Author contribution(s): Wenting Yan: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft.
Xiujuan Wu: Funding acquisition; Investigation; Methodology; Software; Writing – original draft.
Shushu Wang: Investigation; Resources; Validation.
Cheng He: Investigation; Resources; Software.
Ling Zhong: Investigation; Resources; Software; Validation.
Peng Tang: Project administration; Validation; Writing – review & editing.
Lin Ren: Formal analysis; Investigation; Supervision; Validation.
Ting Zhang: Resources; Software.
Xiaowei Qi: Conceptualization; Funding acquisition; Project administration; Supervision; Writing – review & editing.
Yi Zhang: Conceptualization; Funding acquisition; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The present study was funded by the Clinical Research Funding of Southwest Hospital (Grant no. SWH2015LC16); the Military Medical Staff Innovation Plan of Southwest Hospital (Grant no. SWH2018BJLC-04); the National Science Foundation for Young Scientists of China (Grant no. 81802665); and the Foundation of Army Medical University (Grant no. XZ-2019-505-042).
Conflict of interest statement: The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Wenting Yan, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Xiujuan Wu, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Shushu Wang, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Cheng He, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Ling Zhong, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Peng Tang, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Lin Ren, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Ting Zhang, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Chongqing, China.
Xiaowei Qi, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Gaotanyan Street 29, Chongqing 400038, China.
Yi Zhang, Department of Breast and Thyroid Surgery, Southwest Hospital, Third Military Medical University (Army Medical University), Gaotanyan Street 29, Chongqing 400038, China.
References
- 1. Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. New Engl J Med 2010; 363: 1938–1948. [DOI] [PubMed] [Google Scholar]
- 2. Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov 2019; 9: 176–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chaudhary LN. Early stage triple negative breast cancer: management and future directions. Semin Oncol 2020; 47: 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clinical Oncol 2017; 35: 1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet (London, England) 2014; 384: 164–172. [DOI] [PubMed] [Google Scholar]
- 6. Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clinical Oncol 2008; 26: 1275–1281. [DOI] [PubMed] [Google Scholar]
- 7. von Minckwitz G, Schneeweiss A, Loibl S, et al. Neoadjuvant carboplatin in patients with triple-negative and HER2-positive early breast cancer (GeparSixto; GBG 66): a randomised phase 2 trial. Lancet Oncol 2014; 15: 747–756. [DOI] [PubMed] [Google Scholar]
- 8. Gluz O, Nitz U, Liedtke C, et al. Comparison of neoadjuvant nab-paclitaxel+carboplatin vs nab-paclitaxel+gemcitabine in triple-negative breast cancer: randomized WSG-ADAPT-TN trial results. J Nat Can Inst 2018; 110: 628–637. [DOI] [PubMed] [Google Scholar]
- 9. Lee JS, Yost SE, Yuan Y. Neoadjuvant treatment for triple negative breast cancer: recent progresses and challenges. Cancers (Basel) 2020; 12: 1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silver DP, Richardson AL, Eklund AC, et al. Efficacy of neoadjuvant Cisplatin in triple-negative breast cancer. J Clinical Oncol 2010; 28: 1145–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hurley J, Reis IM, Rodgers SE, et al. The use of neoadjuvant platinum-based chemotherapy in locally advanced breast cancer that is triple negative: retrospective analysis of 144 patients. Breast Cancer Res Treat 2013; 138: 783–794. [DOI] [PubMed] [Google Scholar]
- 12. McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs 2001; 10: 119–128. [DOI] [PubMed] [Google Scholar]
- 13. Gietema JA, de Vries EG, Sleijfer DT, et al. A phase I study of 1,2-diamminomethyl-cyclobutane-platinum (II)-lactate (D-19466; lobaplatin) administered daily for 5 days. Br J Cancer 1993; 67: 396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deng QQ, Huang XE, Ye LH, et al. Phase II trial of Loubo® (Lobaplatin) and pemetrexed for patients with metastatic breast cancer not responding to anthracycline or taxanes. Asian Pac J Cancer Prev 2013; 14: 413–417. [DOI] [PubMed] [Google Scholar]
- 15. Harstrick A, Bokemeyer C, Scharnofkse M, et al. Preclinical activity of a new platinum analogue, lobaplatin, in cisplatin-sensitive and -resistant human testicular, ovarian, and gastric carcinoma cell lines. Cancer Chemother Pharmacol 1993; 33: 43–47. [DOI] [PubMed] [Google Scholar]
- 16. Lv X, Cao X, Xia WX, et al. Induction chemotherapy with lobaplatin and fluorouracil versus cisplatin and fluorouracil followed by chemoradiotherapy in patients with stage III-IVB nasopharyngeal carcinoma: an open-label, non-inferiority, randomised, controlled, phase 3 trial. Lancet Oncol 2021; 22: 716–726. [DOI] [PubMed] [Google Scholar]
- 17. Isakoff SJ. Triple-negative breast cancer: role of specific chemotherapy agents. Cancer J 2010; 16: 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gietema JA, Veldhuis GJ, Guchelaar HJ, et al. Phase II and pharmacokinetic study of lobaplatin in patients with relapsed ovarian cancer. Br J Cancer 1995; 71: 1302–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Long GX, Lin JW, Liu DB, et al. Single-arm, multi-centre phase II study of lobaplatin combined with docetaxel for recurrent and metastatic nasopharyngeal carcinoma patients. Oral Oncol 2014; 50: 717–720. [DOI] [PubMed] [Google Scholar]
- 20. Wu X, Tang P, Li S, et al. A randomized and open-label phase II trial reports the efficacy of neoadjuvant lobaplatin in breast cancer. Nat Commun 2018; 9: 832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clinical Oncol 2010; 28: 2784–2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clinical Oncol 2013; 31: 3997–4013. [DOI] [PubMed] [Google Scholar]
- 23. Sharma P, López-Tarruella S, García-Saenz JA, et al. Efficacy of neoadjuvant carboplatin plus docetaxel in triple-negative breast cancer: combined analysis of two cohorts. Clin Cancer Res 2017; 23: 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma P, López-Tarruella S, García-Saenz JA, et al. Pathological response and survival in triple-negative breast cancer following neoadjuvant carboplatin plus docetaxel. Clin Cancer Res 2018; 24: 5820–5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poggio F, Bruzzone M, Ceppi M, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol 2018; 29: 1497–1508. [DOI] [PubMed] [Google Scholar]
- 26. Sharma P, Kimler BF, O’Dea A, et al. Randomized Phase II Trial of Anthracycline-free and Anthracycline-containing Neoadjuvant Carboplatin Chemotherapy Regimens in Stage I-III Triple-negative Breast Cancer (NeoSTOP). Clin Cancer Res 2021; 27: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu L, Wang B, Gao M, et al. Intrapleural combination therapy with lobaplatin and erythromycin for non-small cell lung cancer-mediated malignant pleural effusion. Thoracic Cancer 2018; 9: 950–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shan L, Bai B, Lv Y, et al. Lobaplatin suppresses proliferation and peritoneal metastasis of colorectal cancer in a preclinical model. Biomed Pharmacother 2018; 108: 486–491. [DOI] [PubMed] [Google Scholar]
- 29. Wang Z, Xu L, Wang H, et al. Lobaplatin-based regimens outperform cisplatin for metastatic breast cancer after anthracyclines and taxanes treatment. Saudi J Biol Sci 2018; 25: 909–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tarantino P, Gandini S, Trapani D, et al. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: a systematic review and meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol 2021; 159: 103223. [DOI] [PubMed] [Google Scholar]
- 31. Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. New Engl J Med 2020; 382: 810–821. [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Li S, Qi J, et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis 2019; 10: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tian W, Hao S, Gao B, et al. Lobaplatin inhibits breast cancer progression, cell proliferation while it induces cell apoptosis by downregulating MTDH expression. Drug Des Devel Ther 2018; 12: 3563–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ke LR, Xia WX, Qiu WZ, et al. Safety and efficacy of lobaplatin combined with 5-fluorouracil as first-line induction chemotherapy followed by lobaplatin-radiotherapy in locally advanced nasopharyngeal carcinoma: preliminary results of a prospective phase II trial. BMC Cancer 2017; 17: 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen MQ, Chen C, Lu HJ, et al. The efficacy and toxicities of combined lobaplatin with paclitaxel as a first-line chemotherapy for advanced esophageal squamous cell carcinoma. J Thorac Dis 2015; 7: 1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221107111 for Lobaplatin-based neoadjuvant chemotherapy for triple-negative breast cancer: a 5-year follow-up of a randomized, open-label, phase II trial by Wenting Yan, Xiujuan Wu, Shushu Wang, Cheng He, Ling Zhong, Peng Tang, Lin Ren, Ting Zhang, Xiaowei Qi and Yi Zhang in Therapeutic Advances in Medical Oncology