Abstract
Background:
Regular alcohol consumption, e.g. by social drinking, is a potential source of consecutive health problems in many countries worldwide. A probiotic nutritional supplement (AB001) has been developed to reduce alcohol absorption from the intestine tract and to mitigate potential health care risks.
Methods:
This randomized placebo-controlled double-blind crossover study was conducted with 24 healthy subjects (13 male, 11 female, age: 25.4 ± 7.7 years, BMI: 23.6 ± 2.5 kg/m²). The subjects were randomized to take 2 capsules/day of AB001 or placebo for 1 week prior to an alcohol exposure experiment. On the experimental day, they ingested a light breakfast and drank a moderate glass of spirit (0.3 g/kg body weight). Breath alcohol tests and blood draws for determination of blood alcohol levels were performed for up to 6 hours. After crossover, the experiment was repeated in the following week. Areas under the curves were calculated to determine alcohol absorption rates.
Results:
A significant reduction of blood alcohol levels by 70.3% (P < 0.005 vs. placebo) was seen with AB001, (breath test: −30.7%; P < 0.005 vs. placebo). No difference was seen in a cognitive function test performed 60 minutes after alcohol ingestion (22.4 ± 7.7 seconds vs. 22.7 ± 5.6 seconds, n.s.). There were no adverse events or serious adverse events reported in this study
Conclusions:
One week of supplementation with AB001 resulted in a substantially reduced absorption of alcohol into the body. Regular uptake of AB001 may help to prevent liver and other organ damage, and may reduce the negative medical and economical impact of social drinking on the individual and the society.
Keywords: Probiotic nutritional supplement, alcohol absorption, alcohol degradation, liver protection
Introduction
Over centuries, alcohol has become the most socially-accepted addictive drug worldwide, and drinking alcoholic beverages is a common feature of social gatherings. 1 It is accepted as a legitimate way to celebrate special occasions or just to relax after a hard day at work. Drinking in moderation tends to be viewed as a harmless activity. It is only those who are habitually intoxicated who get judged as engaging in dangerous behavior. In reality, there is no level of alcohol use that can be considered completely risk free. It is known and accepted that light to moderate alcohol consumption is beneficial to cardiovascular health, but heavy drinking often results in organ damage and social problems.2 -4 While social drinking is commonly accepted, alcohol abuse is a serious medical and social problem. It has been shown that alcohol use (and quantity of use) and chronic health conditions are significant contributors to varying levels of emergency department visit risk in late middle-aged and older adults. 5 But at the end, almost 3.3 million people die from alcohol abuse each year worldwide accounting for almost 5% of all deaths. 4
Postulated pathomechanisms resulting in alcoholic organ injuries and diseases, include, but are not limited to direct toxicity of ethanol and its main metabolite acetaldehyde, alcohol-induced oxidative stress, accumulation of fatty acid ethyl esters and modification of lipoprotein and apolipoprotein particles.1,6 Fatty acid ethyl esters, have been shown to contribute to damage to the heart, liver and pancreas.7,8 The most important organ for alcohol metabolism is the liver, 9 but other organs including the heart, pancreas, gastrointestinal tract, and the brain, also participate in ethanol degradation and metabolism to form acetaldehyde.7 -10 Acetaldehyde causes mitochondrial dysfunction and compromises acetaldehyde metabolism leading in turn in the accumulation of acetaldehyde—a vicious cycle. In addition, it reacts with amino, hydroxyl, and sulfhydryl groups and interferes or modifys the structure and function of proteins and enzymes in the body.8,11
It is well known though that alcohol drinking-induced effects may exhibit large individual variation. Therefore, it is very difficult to figure out for the individual person where the line is between social drinking and problem drinking. In any case, chronic alcohol ingestion or binge drinking may trigger detrimental organ damage, which is also influenced by many factors, such as genetics, race, emotional health, and the environment. 1 To avoid alcohol damage, it is essential to understand the drinking problems, the definition of moderate drinking, alcoholism and alcohol abuse. Strategies to minimize the harmful consequences of alcohol abuse are focusing on protective general strategies based on, that is, “Limits” or limiting consumption, and “Avoidance” or avoiding alcohol in general or specific alcohol situations. However, the social context (i.e., drinking with friends) predicts elevated drinking, but is unrelated to use of preventive behavioral strategies. 12
Under such circumstances, access to a safe method discretely inhibiting the absorption of the ingested alcohol into the blood may be a helpful way to prevent alcohol-induced organ damage. de Faire Medical AB (Stockholm, Sweden, www.defairemedical.com) has developed a probiotic nutritional supplement, which is composed of Bacillus strains. The bacteria are released from acid-resistant capsules and settle in the upper part of the intestine tract. Here, they stay for about 1 day before being eliminated from the body through the feces. The bacterial strains were selected to preferably and effectively metabolize ethyl alcohol into CO2 and water, thus reducing the further resorption of alcohol from the intestine tract. In consequence, less alcohol is expected to be absorbed by the body, and damage of organs through alcohol degradation products is expected to be diminished.
The purpose of this study was to evaluate the decrease in alcohol absorption into the blood and the breath alcohol levels when drinking a defined amount of alcohol after 1 week of dietary supplementation with either AB001 or placebo in healthy subjects. In addition, cognitive function 1 hour after the alcohol uptake and the tolerability of both interventions was explored.
Subjects and Methods
The study was conducted in accordance with Good Clinical Practice guidelines and international and local ethical and scientific standards. The protocol was approved by the responsible ethical review board (Landesärztekammer Rheinland-Pfalz, Mainz, Germany), registered with the responsible national authority (Bundesamt für Verbraucherschutz und Lebenmittelsicherheit) and registered at the German Clinical Trial Registry (DRKS00023744). Prior to participation, all subjects signed written informed consent.
The study compounds
AB001 consists of naturally fermented rice bran containing Bacillus subtilis, B. coagulans, L-Cysteine and Dextrin (excipients: magnesium stearate salts, calcium phosphate and potassium phosphate) Contents/dose (800 mg): fermented rice bran 560 mg, L-Cysteine 200 mg, dextrin 4 mg and excipients 36 mg. The placebo formulation contained 800 mg of rice flour.
Study procedure
Primary objective was the impact of AB001 on plasma levels of alcohol after uptake of a high alcoholic beverage (as compared to placebo) after 7 days of nutritional intervention. Secondary objectives were the impact of AB001 on breath alcohol test results and cognitive function as well as the tolerability of the probiotic supplement, as assessed by the number and type of documented (serious) adverse events.
To be eligible for the study, a healthy subject had to be male or female and older than 18 years, of Caucasian ethnicity, and not suffering from any major disease. Main exclusion criteria were present (or history of) alcohol addiction, any acute or chronic disease, known allergies against probiotic nutritional supplements, pregnancy or breast feeding, or regular uptake of any other nutritional supplement. The subjects were not allowed to drink alcohol in the 7 days prior to the experiments.
At the screening visit, blood was drawn for the safety analysis and for identification of potential exclusion criteria, and the randomization into the two study arms took place.
Thereafter, the enrolled subjects were asked to participate in two experimental procedures after 1 week each of regular administration of two capsules per day of placebo or AB001. After arrival at the study site, they were eating a light breakfast, and thereafter they ingested 0.3 g alcohol/kg body weight in a high alcoholic spirit (wodka). A breath test (Dräger Alcotest 3820, Dräger Safety AG, Lübeck, Germany) and a blood draw (NaF whole blood) for measurement of alcohol in a central laboratory (photometric Cer(IV)ammoniumnitrate/HNO3 method, Labor Augsburg MVZ GmbH, Augsburg, Germany) at timepoints 0 minute, 15 minute, 30 minute, 45 minute, 60 minute, 90 minute, 120 minute, 180 minute, 240 minute, 300 minute, and 360 minute. Prior to drinking the alcohol and after 60 minute, the participants were asked to perform a number connection test. The time required for completion of the test was documented. The experiment was run for at least 120 min, and until no alcohol was seen in the breath test at two consecutive timepoints. After the second experiment, the patients were discarded from the study.
Number connection tests (NCT) have been used for many decades to assess mental performance. 13 The NCT measures cognitive processing speed and is involving psychomotor responding. The participant receives a sheet of paper with 25 numbers and is asked to draw a continuous line from number 1 to number 25, connecting all numbers in between without elevating the pencil from the paper. The time will be recorded in seconds. In case of a mistake, the investigator/study personnel will ask the participant to correct the mistake without stopping the time.
The data was evaluated using methods of standard exploratory and descriptive analyses to gain an understanding of the qualitative and quantitative nature of the collected data. For quantitative variables arithmetic means, medians, standard deviations, and minimum and maximum values were presented. Appropriate parametric and non-paramteric statistical tests were used to compare the collected results. Students t-test was used to compare the areas under the curve of the blood and breath alcohol concentrations measured during the two exposure experiments. A P-value < 0.05 was considered to be statistically significant.
Results
As planned, 24 healthy subjects were enrolled into the study (13 men, 11 women, mean age: 25.4 ± 7.7 years (range: 18 years–55 years), BMI: 23.6 ± 2.5 kg/m² (range: 19.1 kg/m²–29.1 kg/m²). All participants performed the study per protocol and were included into the safety and efficacy analysis. There were no systematic differences, for example, with respect to baseline blood or breath aclohol levels between the two experimental days. In general, the amount of alcohol ingested was low (47 mL to 89 mL of a spirit containing 40% of alcohol), and did not result in measurable blood alcohol concentrations with any of the two interventions in 6 subjects (25%). From the remaining participants, 4 did not show a blood alcohol concentration with AB001 or placebo above 0.1 o/oo (>0.124 g/L; 17% of the global population). Breath alcohol levels above 0.1 o/oo (>0.124 g/L) were detected in 18 subjects (75%).
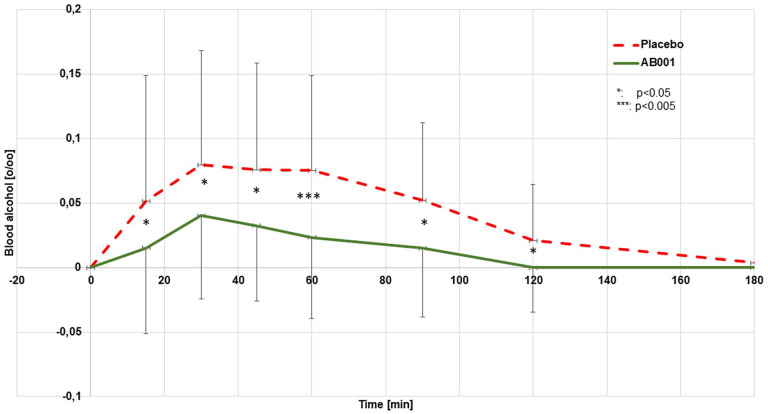
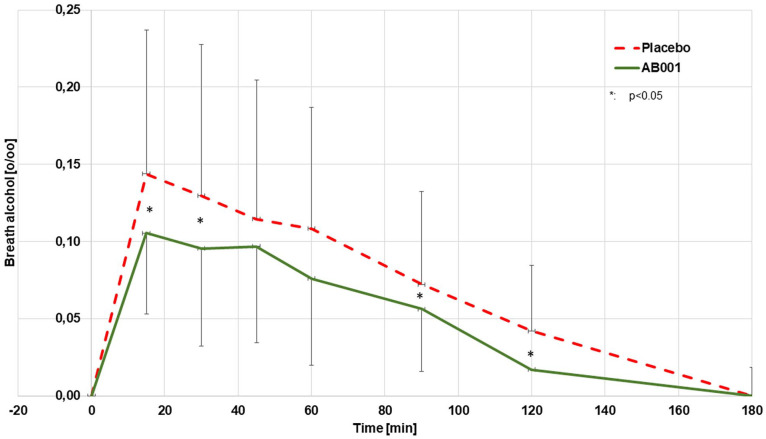
The mean blood concentrations after 1 week of regular nutritional supplementation with 2 capsules/day of study product or placebo are provided in Figure 1. There was no measurable alcohol level in the blood detectable beyond 180 minutes in any of the individual experiments. The AUCBlood (0–180 minutes) was calculated to be 8.5 ± 0.6 o/oo*minutes (10.6 ± 0.7 g/L*minutes) from the placebo experiments and 2.5 ± 0.2*minutes (3.1 ± 0.3 g/L*minutes) from the verum experiments (−70.3%; P < 0.005). The mean breath alcohol concentrations measured in parallel are provided in Figure 2. There was no measurable alcohol level in the breath detectable beyond 180 minutes in any of the individual experiments. The AUCBreath (0–180 minute) as calculated from the mean concentrations was 14.0 o/oo*mL (17.4 g/L*minutes) from the placebo experiments and 9.7 o/oo*mL (12.0 g/L*minute) from the verum experiments (−30.7 %, P < 0.005).
Figure 1.
Mean blood alcohol concentrations after oral uptake of alcohol (0.3 g/kg body weight, n = 24).
Figure 2.
Mean breath alcohol concentrations after oral uptake of alcohol (0.3 g/kg body weight, n = 24).
The uptake of 0.3 g/kg bodyweight of alcohol did not impact the cognitive function of the patients as assessed by measuring the time required to complete the standardized number connection test. No difference was seen in the cognitive function test performed at baseline (AB001 vs. Placebo: 21.6 ± 6.8 vs. 23.0 ± 8.2, n.s.) or 60 minutes after alcohol ingestion (22.4 ± 7.7 seconds vs. 22.7 ± 5.6 seconds, n.s.).
The nutritional supplements were well tolerated, and there were no adverse events or serious adverse events reported in this trial. In addition, there were no clinically relevant deviations from normal values observed in the safety biochemistry panels when taken before and after the study.
Discussion
Probiotics are live microorganisms promoted with claims that they provide health benefits when consumed, generally by improving or restoring the gut flora. Probiotics have been shown to be effective in varied clinical conditions- ranging from infantile diarrhea, necrotizing enterocolitis, antibiotic-associated diarrhea, relapsing Clostridium difficile colitis, Helicobacter pylori infections, inflammatory bowel disease to cancer, female urogenital infection, and surgical infections. 14 Probiotics have been used to prevent and treat diseases associated with gut-derived bacterial products and disorders associated with gut leakiness. Indeed, “probiotic” Lactobacillus has been successfully used to treat alcohol-induced liver injury in rats. This improvement was associated with reduced markers of intestinal and liver oxidative stress and inflammation and preserved gut barrier function. 15 These animal studies provide a scientific rationale to test probiotics for treatment and/or prevention of alcoholic liver disease in man. In this study, we employed a nutritional supplement (AB001), which is predominantly composed of Bacilli species (13 million cfu/g), fermented rice bran, dextrin, fatty acid magnesium salts and calcium phosphate. The bacterial strains were selected to preferably and effectively metabolize ethyl alcohol into CO2 and water, thus reducing the further resorption of alcohol from the intestine tract. Another mechanism of action may be a reduction of intestinal oxidative stress, which normalizes the barrier function of the intestinal mucosa and may result in less alcohol absorption. 16
In our study, a substantial reduction of alcohol absorption into the blood by more than 70% was observed after 1 week of AB001 supplementation as compared to placebo. The reduction of measurable alcohol in the breath was also reduced significantly but to a lower extent (by ~30%). It is well known that breath and blood alcohol have a high correlation, but the results of breath-alcohol measurement are more prone to physiological variations such as body and breath temperature, pulmonary function, and pattern of breathing prior to exhalation. 17 It is therefore well possible that the final alcohol content in breath is normally more driven by the alcohol that is early absorbed in the upper ingestion tract, that is, in the buccal mucosa and in the stomach. This would explain, why the measured impact with AB001, which is only active in the intestine tract, was more pronounced in the blood tests than in the breath tests.
One limitation of our study is that the amount of alcohol ingested (0.3 g/kg body weight) did not lead to measurable relevant alcohol concentrations in the blood in 10 cases (42%), and the data of these individuals could therefore ultimately only be used for the safety and tolerability analysis. The amount of alcohol to be administered in this study was defined by the IRB in the study approval process. It should be considered in future studies that a higher amount of alcohol uptake may be required to investigate the effect of AB001 in an appropriate fashion. This notion is also underlined by the finding that the ingested amount of alcohol did not influence cognitive function in any way. In a placebo-controlled follow-up study (data not shown), we showed that the impact of a single dose of AB001 taken prior to drinking twice the amount of alcohol still resulted in a significant reduction of blood and breath alcohol levels but to a lesser extend (−10% and −7%, respectively). Taken together these findings suggest that a better effect can be achieved if the bacteria contained in AB001 are given sufficient time and dosing support to proper settle in the intestine tract.
Another limitation is the small study size with a lot of age variation in the test subjects, and the fact that finally only data from 14 subjects could be included into the efficacy analysis. We have tried to minimize the impact of this variability by means of the cross-over study design. By this design, each parcticipant serves as his/her own control, which practically eliminates the majority of potential interfering conditions.
Conclusion
One week of regular supplementation with AB001 resulted in a substantially lower uptake of alcohol into the blood and hence into the metabolism. Intake of AB001 as a nutritional supplement may therefore help to prevent liver and other organ damage known to be associated with regular alcohol uptake and may reduce the negative medical and economical impact of social drinking on the individual and the society. It is important to emphasize that this product is not intended to be used in the treatment of alcohole abuse. Primarily the aim of this pilot study was to scientifically demonstrate an interesting effect of this probiotic compound, which may be of potential importance for human health.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study reported in this manuscript was funded by DeFaire Medical AB, Stockholm, Sweden.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The study reported in this manuscript has been funded by DeFaire Medical AB. APF has received a research grant for this project from deFaire Medical. JDEF is founder and shareholeder of deFaire Medical AB.
References
- 1. Guo R, Ren J. Alcohol and acetaldehyde in public health: from marvel to menace. Int J Environ Res Public Health. 2010;7:1285-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gaziano JM, Gaziano TA, Glynn RJ, et al. Light-to-moderate alcohol consumption and mortality in the physicians’ Health Study enrollment cohort. J Am Coll Cardiol. 2000;35:96-105. [DOI] [PubMed] [Google Scholar]
- 3. Klatsky AL, Friedman GD, Siegelaub AB. Alcohol and mortality. A ten-year Kaiser-Permanente experience. Ann Intern Med. 1981;95:139-145. [DOI] [PubMed] [Google Scholar]
- 4. Shramko SS, Golina VV, Kolodyazhny MG. Alcoholism as a medical and socio-legal problem and ways to solve it. Wiad Lek. 2019;72:2496-2500. [PubMed] [Google Scholar]
- 5. Choi NG, Marti CN, DiNitto DM, Choi BY. Alcohol use as risk factors for older adults’ emergency department visits: A Latent Class analysis. West J Emerg Med. 2015;16:1146-1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hannuksela ML, Liisanantti MK, Savolainen MJ. Effect of alcohol on lipids and lipoproteins in relation to atherosclerosis. Crit Rev Clin Lab Sci. 2002;39:225-283. [DOI] [PubMed] [Google Scholar]
- 7. Vonlaufen A, Wilson JS, Pirola RC, Apte MV. Role of alcohol metabolism in chronic pancreatitis. Alcohol Res.Health. 2007;30:48-54. [PMC free article] [PubMed] [Google Scholar]
- 8. Ren J, Wold LE. Mechanisms of alcoholic heart disease. Ther Adv Cardiovasc Dis. 2008;2:497-506. [DOI] [PubMed] [Google Scholar]
- 9. Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res. Health. 2006;29:245-254. [PMC free article] [PubMed] [Google Scholar]
- 10. Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5-13. [PMC free article] [PubMed] [Google Scholar]
- 11. Lieber CS, DeCarli LM, Feinman L, et al. Effect of chronic alcohol consumption on ethanol and acetaldehyde metabolism. Adv Exp Med Biol. 1975;59:185-227. [DOI] [PubMed] [Google Scholar]
- 12. Braitman AL, Linden-Carmichael AN, Henson JM. Protective behavioral strategies as a context-specific mediator: A multilevel examination of within- and between-person associations of daily drinking. Exp Clin Psychopharmacol. 2017;25:141-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lezak MD, Howieson DB, Loring DW, eds. Neuropsychological Assessment; 4th Ed. Oxford University Press; 2004. [Google Scholar]
- 14. Gupta V, Garg R. Probiotics. Indian J Med Microbiol. 2009;27:202-209. [DOI] [PubMed] [Google Scholar]
- 15. Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol. 2009;43:163-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao Y, Drabik KA, Waypa TS, et al. Soluble factors from Lactobacillus GG activate MAPKs and induce cytoprotective heat shock proteins in intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C1018-C1030. [DOI] [PubMed] [Google Scholar]
- 17. Jones AW. Medicolegal alcohol Determination - blood- or breath-alcohol concentration? Forensic Sci. Rev. 2000;12:23-47. [PubMed] [Google Scholar]