Fig. 3.
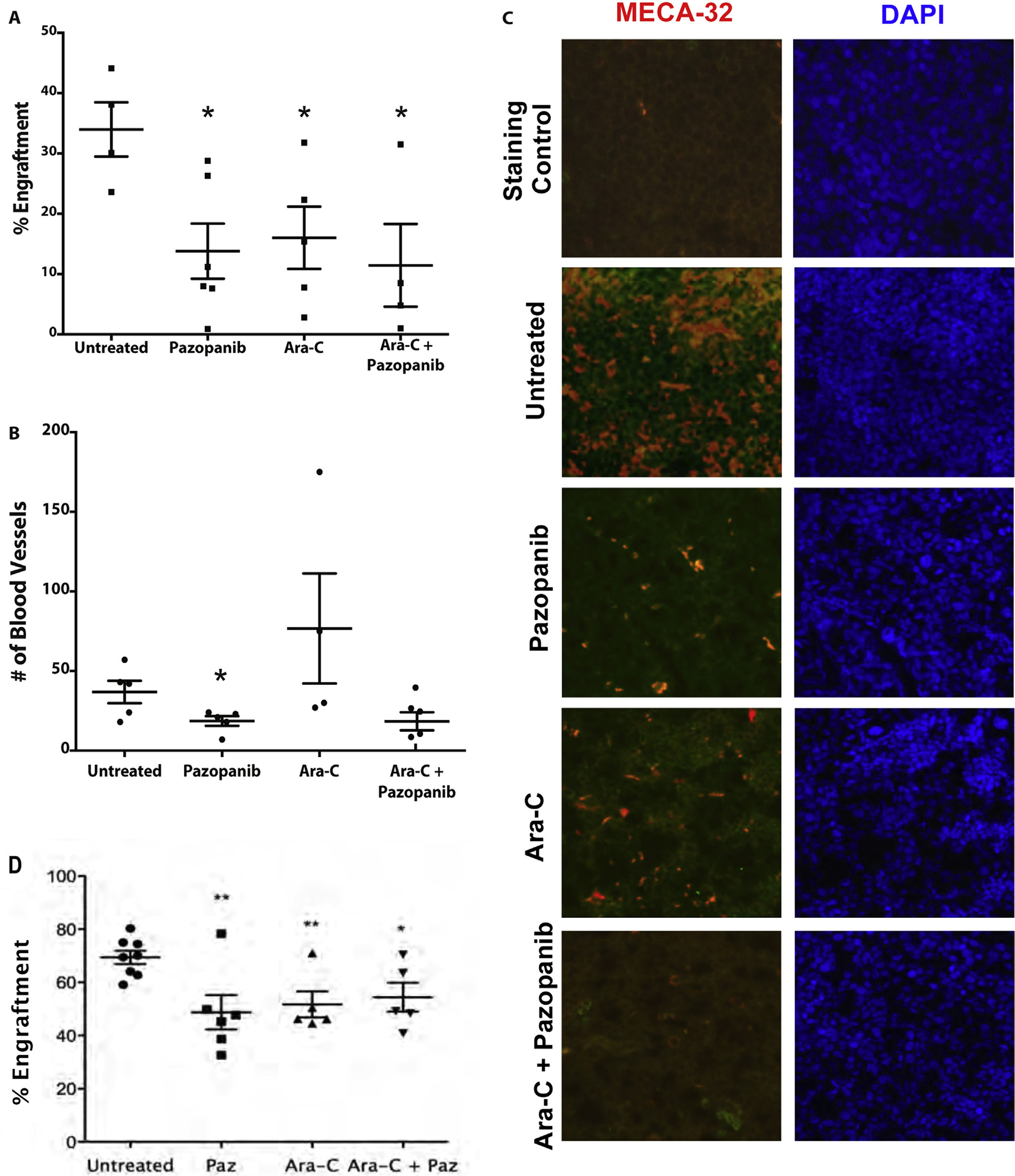
Pazopanib treatment decreases engraftment equivalent to cytarabine and disrupts microvessel formation. (A) Pazopanib and cytarabine decreased the level of HL-60 engraftment equally. The decrease in engraftment for all treatment groups was determined by flow cytometry analysis. HL-60 engraftment for untreated mice was approximately 34%. HL-60 engraftment for treated mice ranged 12–18%. *p-values <0.05 for all treatment samples compared to untreated control. (B) Pazopanib treatment leads to a reduction in the number of blood microvessels within leukemic cores compared to other treatment groups. Quantification of microvessels based on MECA-32+. Values represent mean ± SEM. p < .05. (C) Representative bone marrow micrographs showing microvessel density (MECA-32 endothelial stain, red) and nuclear staining (DAPI, blue) of mouse femurs after transplant with human AML cells (HL-60) and treatment with pazopanib and cytarabine. Untreated animals with robust AML engraftment showed high microvessel density in the bone marrow. Pazopanib alone markedly decreased microvessel density in the bone marrow. Cytarabine also decreased microvessel density. The combination of pazopanib and cytarabine also caused marked reduction in blood vessels within bone marrow. (D) Pazopanib and cytarabine decreased levels of bone marrow KG1 engraftment equally. The decrease in engraftment for all treatment groups was determined by flow cytometry analysis. KG1 engraftment for untreated mice ranged 59–80%. KG1 engraftment for treated mice ranged 33–78%. **p-values <0.005, *p-value <0.05 for all treatment samples compared to untreated control.
